ENGR 1A Lecture Notes - Lecture 8: Trigonal Bipyramidal Molecular Geometry, Chemical Polarity, Valence Electron
ENGR 1A verified notes
8/31View all
7

ENGR 1A Lecture Notes - Lecture 7: Trigonal Planar Molecular Geometry, Electron Shell, Valence Electron
8
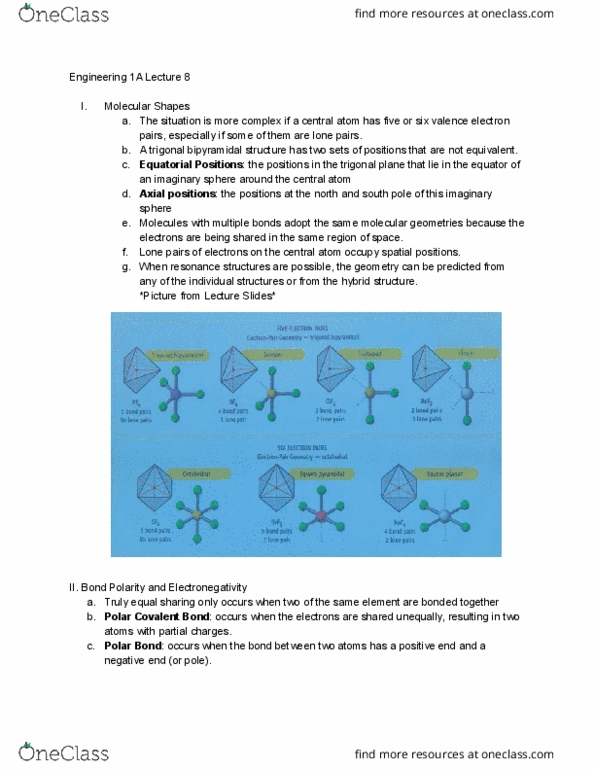
ENGR 1A Lecture Notes - Lecture 8: Trigonal Bipyramidal Molecular Geometry, Chemical Polarity, Valence Electron
9

ENGR 1A Lecture Notes - Lecture 9: Valence Bond Theory, Linus Pauling, Covalent Bond
Document Summary
: the positions in the trigonal plane that lie in the equator of: axial positions , molecules with multiple bonds adopt the same molecular geometries because the. Bond polarity and electronegativity: truly equal sharing only occurs when two of the same element are bonded together, polar covalent bond , polar bond . : occurs when the bond between two atoms has a positive end and a. : occurs when the electrons are shared unequally, resulting in two atoms with partial charges. negative end (or pole): electronegativity . If the difference is less than 1. 8 , the bond is polar covalent . If the difference is 0. 5 or less , the bond is covalent polar covalent bond. *picture: http://wikillers. pbworks. com/f/1239212696/electronegativitytrends. gif: electronegativity tells you which atom is going to be partially positive or negative in a. Electroneutrality principle: electroneutrality principle b. c. on all atoms are as close to zero as possible.

