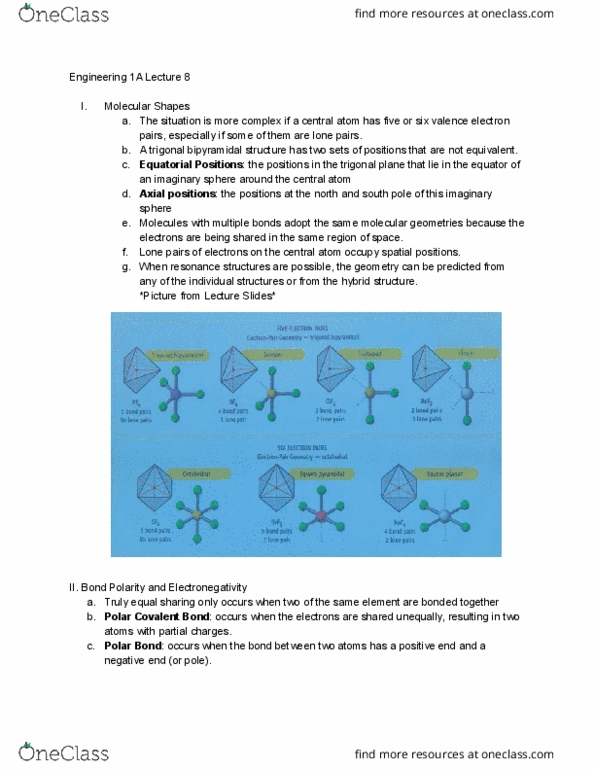
ENGR 1A Lecture Notes - Lecture 7: Trigonal Planar Molecular Geometry, Electron Shell, Valence Electron
ENGR 1A verified notes
7/31View all
Document Summary
Engineering 1a lecture 7: resonance, formal charge, octet rule, and molecular shells. Exceptions to the octet rule: compounds in which an atom has fewer than 8 valence electrons: . Boron compounds that 2 electrons short of the octet can accommodate fourth pair of electrons provided by another atom. Molecules or ions with lone pairs can fulfill this role. the bonded atoms, usually depicted with an arrow: coordinate covalent bond , compounds in which an atom has more than 8 valence electrons . : if a bonding pair of electrons originates on one of. : elements in the 3rd or higher periods often form compounds and ions in which the central element is surrounded by more than 4 valence electron pairs. In most of these cases, the central atom is bound to fluorine, chlorine, or oxygen: molecules with an odd number of valence electrons: .