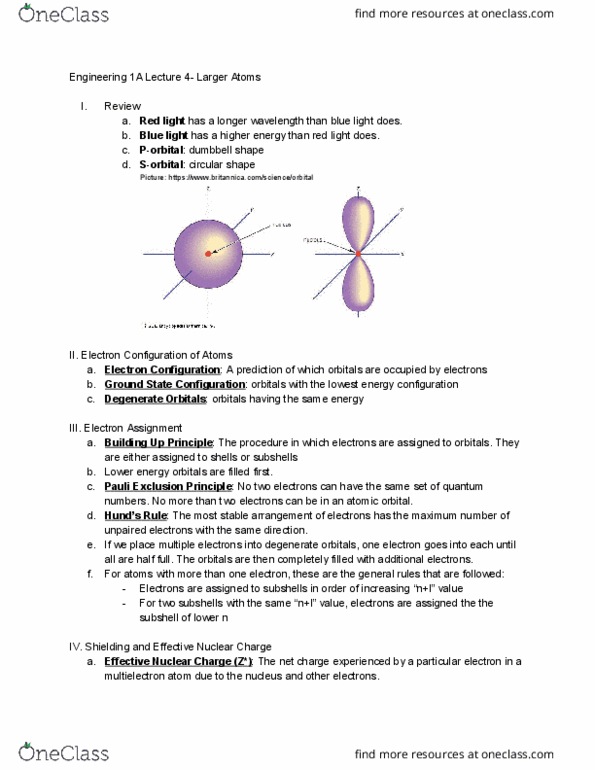
ENGR 1A Lecture Notes - Lecture 5: Effective Nuclear Charge, Valence Electron, Ionization Energy
ENGR 1A verified notes
5/31View all
Document Summary
Engineering 1a lecture 5: periodic properties and bonding. Atomic properties and periodic trends: atomic size: , atomic size generally increases going down a group due to the larger number. : the trend in size changes slightly since electron/electron. : the energy required to remove an electron from an atom in: transition metals repulsions counteract the decrease in size as we go across a period. Atoms other than hydrogen have a series of ionization energies as electrons are removed sequentially (one after the other). e. : positive and negative ions increase in size, but they are: trends in ion sizes , electron affinity h. much smaller and larger than the neutral atoms, respectively. : the energy change for a process in which an electron is acquired by the atom in the gas phase or how bad an atom wants an electron. : when a chemical reaction occurs between two atoms, their valence.