ENGR 1A Lecture Notes - Lecture 11: Orbital Hybridisation, Tetrahedron, Headon
ENGR 1A verified notes
11/31View all
Document Summary
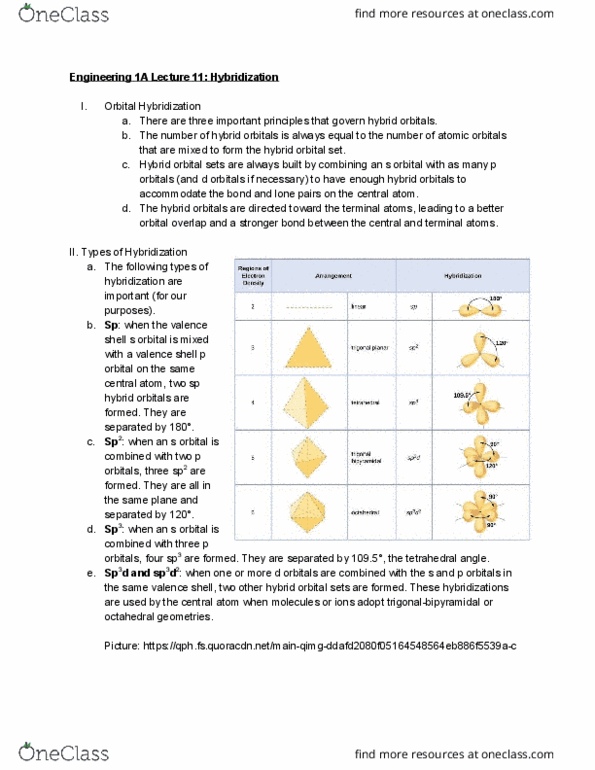
Types of hybridization: the following types of, sp hybridization are important (for our purposes). : when the valence shell s orbital is mixed with a valence shell p orbital on the same central atom, two sp hybrid orbitals are formed. : when an s orbital is combined with two p orbitals, three sp 2 are formed. They are all in the same plane and separated by 120 : sp . : when an s orbital is: sp . 3 combined with three p orbitals, four sp 3 are formed. They are separated by 109. 5 , the tetrahedral angle. : when one or more d orbitals are combined with the s and p orbitals in the same valence shell, two other hybrid orbital sets are formed. These hybridizations are used by the central atom when molecules or ions adopt trigonal-bipyramidal or octahedral geometries. Valence bond theory geometries: trigonal planar geometries .