47
EXPERIMENT
9
Williamson Ether Synthesis
Reading and Preparation
TaskSource
S
-
115
Safety
Chemwatch
(website)
Background
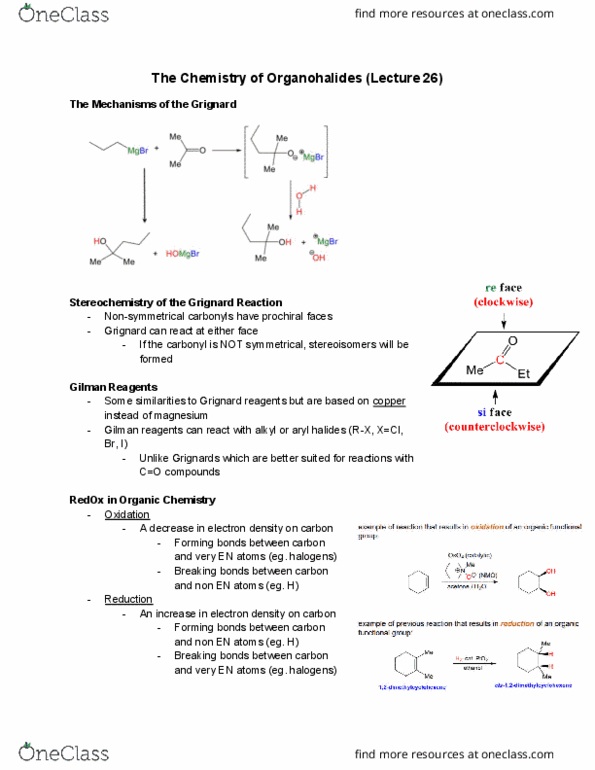
In todayâs laboratory class you will preparetert
-butyl methyl ether from potassiumtert
-
butoxide
and iodomethane using the Williamson ether synthesis according to Scheme 1. This is classified
as a substitution reaction of an alkyl halide, where iodine
in
iodomethane is replaced by a
tert
-
butoxide group.
Scheme 1
Substitution reactions of alkyl halides can occur by either an SN1 or S
N2 mechanism. In the SN1process, the rate-determining step in the reaction is breaking of the carbon-halogen bond and the
formation of a carbocation intermediate (Scheme 2).Scheme 2Such reactions follow first order kinetics because the rate of the reaction only depends on the
decomposition of one molecule and is therefore only affected by the concentration of the alkylhalide. In the SN2 mechanism,
the rate-determining step is the displacement of the halide by an attacking nucleophile (Scheme 3).
Scheme 3
This reaction follows second order kinetics because the rate depends on the interaction between
two molecules and thus the concentration of both the alkyl halide and thetert
-
butoxide
nucleophile. Nucleophilic substitution reactions are discussed in McMurry,
Chapter 11, pages
C
H
I
H
H
+
C
H
3
C
O
H
3
C
H
3
C
C
H
H
H
C
H
3
C
O
H
3
C
H
3
C
K
+
KI
HOC(CH
3
)
3
C
H
H
H
+
I
C
H
I
H
H
C
H
I
H
H
C
H
3
C
O
H
3
C
H
3
C
C
H
3
C
O
H
3
C
H
3
C
C
H
H
H
+
I
48
372
â
395. You will follow the progress of the reaction between potassium
tert
-
butoxide and
iodomethane in order to determine the mechanism of this reaction. If the reaction follows first
order kinetics, then the reaction occurs by the
S
N
1 mechanism. If the reaction follows second
order kinetics, then the reaction occurs by the S
N
2 mechanism.
The progress of the reaction is monitored by following the change in concentration of the
reactants over time. For this experiment, aliquots of the reaction mixture are withdrawn every
10 minutes for the first hour and every 20 minutes for the second hour
. The aliquots of reaction
mixture are placed in cold water to stop the reaction. The unreacted
tert
-
butoxide ions present
reacts with water to liberate hydroxide ions (Scheme 4).
Scheme 4
(H
3
C)
3
CO
Ì
+ H
2
O
â
(H
3
C)
3
COH + HO
Ì
Hydroxide ion is then t
itrated using standardised sulfuric acid and the concentration of the
tert
-
butoxide ion and iodomethane for each time can be calculated.
Notes
:
1.
The concentration of the
potassium
tert
-
butoxide
stated on the stock bottle
cannot be relied
upon as the
solution begins decomposing once prepared. For this reason you will need to
take a 1.00 mL aliquot of the stock solution and quench this in cold water. This solution
will then need to be titrated against the standard H
2
SO
4
solution to get the correct sto
ck
concentration of
tert
-
butoxide
. Note also this is
not
the concentration of the (H
3
C)
3
COK at
time zero in the reaction mixture
â
the (H
3
C)
3
COK solution has been mixed with the CH
3
I
solution in the reaction flask. Thus an appropriate calculation needs t
o be done to get the
(H
3
C)
3
COK concentration in the reaction mixture at time zero.
2.
The concentration stated on the bottle for the stock CH
3
I solution can be used but again note
that the time zero concentration of the CH
3
I in the reaction mixture is not th
is value.
Remember the CH
3
I solution is mixed with (H
3
C)
3
COK solution in the reaction flask so an
appropriate calculation needs to be carried out to get the time zero concentration for the
CH
3
I.
Record the initial concentrations of your two solutions and
the standard sulfuric acid as
shown below and d
raw
a
table in your laboratory notebook
or prepare an excel
spreadsheet
.
Initial Concentration of (H
3
C)
3
COK in (H
3
C)
3
COH
Initial Concentration of CH
3
I in (H
3
C)
3
COH
Concentration of standardised H
2
SO
4
You will use the data calculated in the table to draw 2 graphs. A plot or time versus log [CH
3
I]
will give a straight line if the reaction is first order.
A plot of time versus log ([(H
3
C)
3
CO
Ì
] / [CH
3
I]) will give a straight line if the reaction is second
order.
49
Experimental
*
Note that the melting point of (H
3
C)
3
COH is 23
o
C
â
26
o
C. It may solidify if the room
temperature is cool, so keep all solutions warm.
*
Work in pairs for this experiment.
In separate conical flasks, place iodomethane in
tert
-
butanol (200.0 mL) and potassiumtert
-
butoxide intert
-
butanol (20.0 mL). If not already warmed, place the separate solutions in a water
bath (~40
o
C) for a few minutes. Start
timing on
the stop
-
watch as you quickly combine the
solutions, swirling to mix them thoroughly. Place the reaction vessel in the water bath to
maintain constant temperature. Prepare a conical flask containing cold water (20.0 mL). After
10 minutes pipette an aliquot of th
e reaction mixture (10.0 mL) and run it into the cold water to
quench the reaction. Later you can add phenolphthalein indicator and titrate the liberated
hydroxide ion using the standardised sulfuric acid. After removal of each aliquot the pipette
should
be thoroughly rinsed with water and acetone. The pipette can be dried if necessary by
blowing air through it using the house compressed air and appropriate tubing. Remove aliquots
every 10 minutes for the first hour and every 20 minutes for the second h
our. Write the sulfuric
acid titres in your table, carry out all necessary calculations and plot the graphs.
Questions
1.
By what mechanism would you expect the reaction between potassium
tert
-
butoxide
and iodomethane to proceed? Explain your answer and
write the curved arrow
mechanism.
2.
Do your results support your prediction? If not, offer some possible explanations.
3.
Provide a sample calculation of each of the cells in the spreadsheet for one time
interval. Show the formulae used for each calculation