CHM136H1 Lecture Notes - Lecture 31: Protic Solvent, Leaving Group, Hydrogen Bond
CHM136H1 verified notes
31/39View all
Document Summary
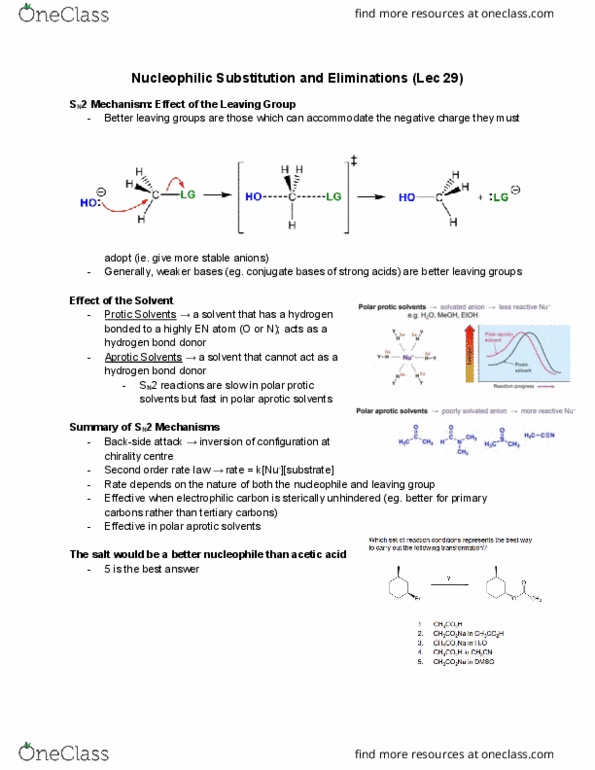
Better leaving groups are those which can accommodate the negative charge they must adopt (ie. give more stable anions) Generally, weaker bases (eg. conjugate bases of strong acids) are better leaving groups. Protic solvents a solvent that has a hydrogen bonded to a highly en atom (o or n); acts as a hydrogen bond donor. Aprotic solvents a solvent that cannot act as a hydrogen bond donor. Sn2 reactions are slow in polar protic solvents but fast in polar aprotic solvents. Back-side attack inversion of configuration at chirality centre. Second order rate law rate = k[nu-][substrate] Rate depends on the nature of both the nucleophile and leaving group. Effective when electrophilic carbon is sterically unhindered (eg. better for primary carbons rather than tertiary carbons) The salt would be a better nucleophile than acetic acid. Step 1: cleavage of the c-br bond to form a carbocation intermediate. Step 2-3: formation of a c-o bond and proton transfer.