saynntan
Siksha 'O' Anusandhan (Deemed to be University)
0 Followers
0 Following
1 Helped
saynntanLv4
26 Mar 2023
Answer: The mass of acetic acid produced by the reaction of 1.6 g of oxygen is...
saynntanLv4
26 Mar 2023
Answer: 5gStep-by-step explanation: The balanced chemical equation for the rea...
saynntanLv4
26 Mar 2023
Answer: 7.37 kJStep-by-step explanation: To calculate the energy given off in ...
saynntanLv4
26 Mar 2023
Answer: ∆H = q / n = 9988 J / 0.2388 mol = 41833 J/mol the molar enthalpy of s...
saynntanLv4
26 Mar 2023
Answer: ΔHrxn = ΔHf°(NaOCl) ΔHrxn/mol = ΔHrxn/2 = ΔHf°(NaOCl)/2 the enthalpy o...
saynntanLv4
26 Mar 2023
Answer: [HOCl][NaN3]/[HN3][NaOCl] [(NH4)2SO4]/[KHSO4][NH3]^2 [K2SO4]/[H2CO3][K...
saynntanLv4
26 Mar 2023
Answer: The [H3O+] and pH of a 0.000413 M hydrazoic acid solution are 2.29×10^...
saynntanLv4
26 Mar 2023
Answer: HN3 + H2O ⇌ H3O+ + N3- pH = 2.72 % dissociation = 0.095% Step-by-step ...
saynntanLv4
26 Mar 2023
Answer: For A) 0.400 M: 1.9x10^-5 = x^2 / (0.4 - x) x = 0.00274 M % ionization...
saynntanLv4
26 Mar 2023
Answer: % ionization = 2.94% For the same concentration of HCl, we would expec...
saynntanLv4
26 Mar 2023
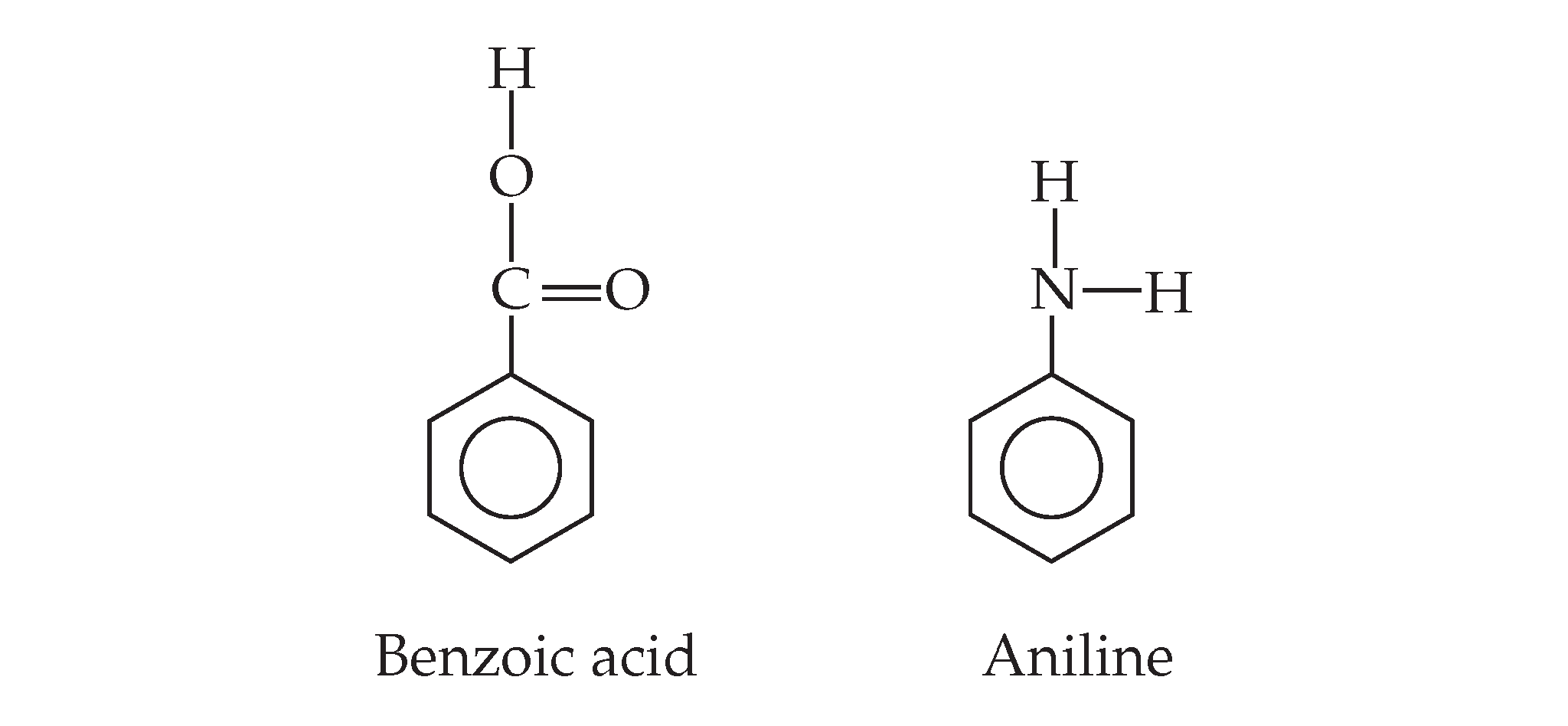
Answer: pH = 8.76% ionization = 100%Step-by-step explanation: Aniline, C6H5NH2...
saynntanLv4
26 Mar 2023
Answer: 10.89Step-by-step explanation: To calculate the pH of aniline (C6H5NH2...
saynntanLv4
26 Mar 2023
Answer: 4.59Step-by-step explanation: Aniline (C6H5NH2) is a weak base, and an...
saynntanLv4
26 Mar 2023
Answer: 3.61Step-by-step explanation: Anilinium chloride, C6H5NH3Cl, is the co...
saynntanLv4
26 Mar 2023
The conjugate base of benzoic acid is the benzoate ion (C6H5COO-), while the c...
saynntanLv4
26 Mar 2023
To determine the LD50 (Lethal Dose 50) of a compound, a series of experiments ...
saynntanLv4
26 Mar 2023
The evolution of technology has had a profound impact on the way people commun...
saynntanLv4
26 Mar 2023
It seems like you're describing how to import and use the math module in Pytho...
saynntanLv4
22 Mar 2023
Answer: Cu(s) + 2Ag⁺(aq) -> Cu²⁺(aq) + 2Ag(s) Reduction: 2Ag⁺(aq) + 2e⁻ -&g...
saynntanLv4
22 Mar 2023
Answer: NaI(aq) + MgSO4(aq) → Na2SO4(aq) + MgI2(aq) Full ionic: 2Na+(aq) + 2I-...
saynntanLv4
22 Mar 2023
Step-by-step explanation: KOH(aq) + Na3PO4(aq) --> no reaction Ionic equati...
saynntanLv4
22 Mar 2023
Step-by-step explanation: To determine the mole ratio of salt to water in a hy...
saynntanLv4
22 Mar 2023
Answer: Theoretical Framework: A theoretical framework refers to the set of co...
saynntanLv4
22 Mar 2023
Answer: 1:10 1:6 1:5 1:2 2:1Step-by-step explanation: The mole ratio of salt t...
saynntanLv4
22 Mar 2023
Answer: 1:10Step-by-step explanation: The mole ratio of salt (Na2SO4) to water...
saynntanLv4
22 Mar 2023
Answer: #include <stdio.h>int main() { float cost, paid, change; int dol...
saynntanLv4
22 Mar 2023
Answer: To purify a solid using recrystallization, a suitable solvent must be ...
saynntanLv4
22 Mar 2023
Answer: The purpose of this second laboratory experiment was to determine a su...
saynntanLv4
22 Mar 2023
Answer: The purpose of this organic chemistry laboratory experiment was to lea...
saynntanLv4
22 Mar 2023
Answer: Pathos: You love your cat and want to give them the best life possible...
saynntanLv4
22 Mar 2023
Answer: Commercial 1 (Pathos): Do you want to be the ultimate host or hostess,...
saynntanLv4
22 Mar 2023
Answer: The low melting point of your pure product could be due to impurities ...
saynntanLv4
22 Mar 2023
Answer: The lower melting point you observed during the recrystallization proc...
saynntanLv4
22 Mar 2023
Answer: products in this experiment: Triphenylmethanol, hydrogen gas, and/or w...
saynntanLv4
22 Mar 2023
Answer: bStep-by-step explanation:Answer: b) Water dissolves in acetone, so ri...
saynntanLv4
22 Mar 2023
Answer: 1. Triphenylmethanol synthesis using the Grignard reaction must be anh...
saynntanLv4
22 Mar 2023
Answer: d c a b dStep-by-step explanation:1. Answer: d. If the glassware is no...
saynntanLv4
22 Mar 2023
Answer: 1. Sublimation of Iodine 2. Solubility Testing 3. Solubility Differenc...
saynntanLv4
22 Mar 2023
Answer: In order to design a procedure to separate the three components of the...
saynntanLv4
22 Mar 2023
Answer: Ether is a type of organic solvent that can be identified through a va...