BIO SCI 93 Lecture 2: BioSci 93 Lecture 2
BIO SCI 93 verified notes
2/31View all
Document Summary
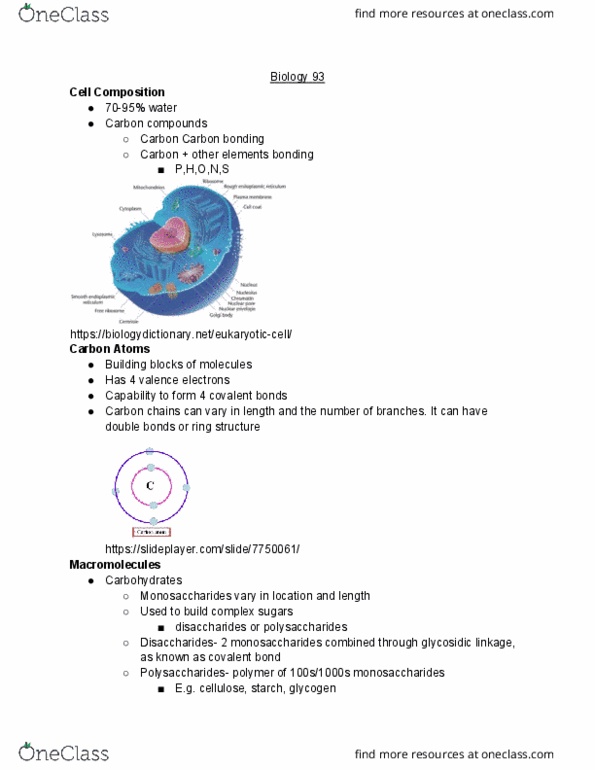
Elements: can t be broken down into other substances. 92 elements, 25 which are essential for life. 96% of living matter is c, o, n, h. Compound: molecule of two or more elements in a fixed ratio. Hydrogen bond: the h atom forms a covalent bond and a second weaker bond with a different atom. Ice has more distance between hydrogen bonds, making it less dense. Cohesion helps water travel up the tree against gravity, hydrogen bonds stick together and pull each other. When a solute is put into a solvent, the water molecules (solvent) surround dissolved ion in hydration shell, dissolving it. Affinity for water: polar or ionic bonds that can form hydrogen bonds with water. Hydrogen ion (h+) and hydroxide ion (oh-) Buffers: resist change in ph of a solution (keep constant) Chemical processes of cells are sensitive to ph changes. Donate hydrogen ions when there is not enough in solution (acids)