CHE 106 Lecture Notes - Lecture 21: Niels Bohr, Emission Spectrum, Continuous Spectrum
CHE 106 verified notes
21/42View all
Document Summary
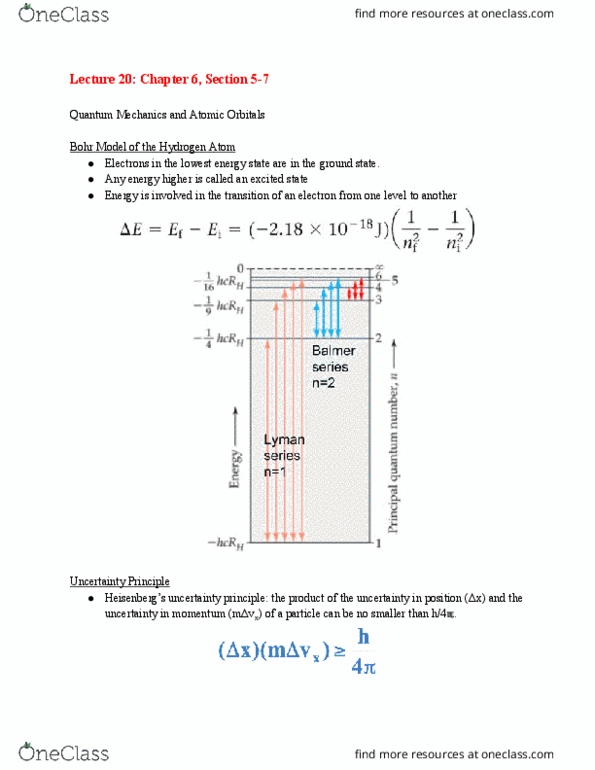
Rutherford gold leaf: alpha particles pass through foil, some deflected at large angles; most of mass of atom concentrated in center. Prior to niels bohr: stability of atom could not be explained using current (at the time) theories. Postulates used to calculate energy of electron and atom. Continuous spectrum: spectrum containing light of all wavelengths. Line spectrum: spectrum showing specific wavelengths of light (only certain colors, or specific range of wavelengths: only line spectrum of discrete wavelengths for atoms and molecules is observed. Jj balmer: showed that the wavelengths in visible spectrum of hydrogen could be reproduced by a simple formula. Light has wave-particle duality (behaves like both a wave and a particle)