CHE 106 Lecture Notes - Lecture 4: Chemical Property, Physical Property, Combustibility And Flammability
52 views17 pages
Verified Note
CHE 106 verified notes
4/42View all
Document Summary
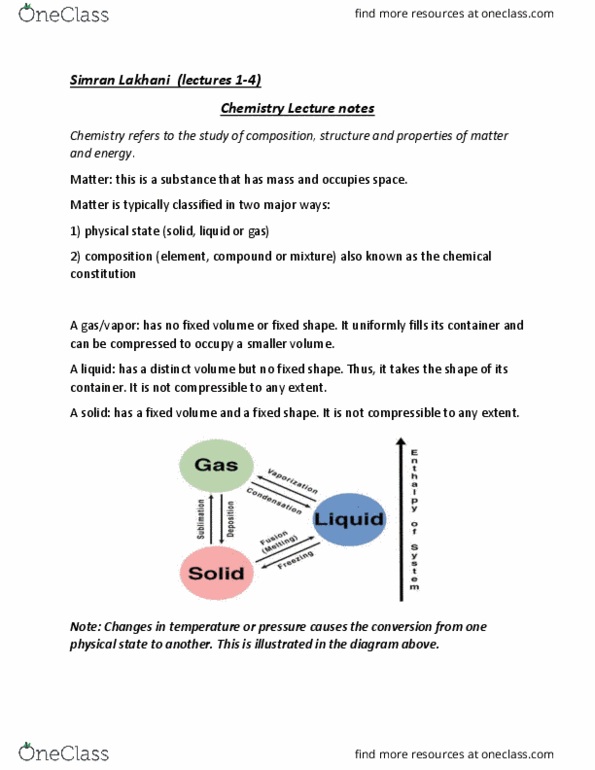
Chemistry refers to the study of composition, structure and properties of matter and energy. Matter: this is a substance that has mass and occupies space. Matter is typically classified in two major ways: physical state (solid, liquid or gas, composition (element, compound or mixture) also known as the chemical constitution. A gas/vapor: has no fixed volume or fixed shape. It uniformly fills its container and can be compressed to occupy a smaller volume. A liquid: has a distinct volume but no fixed shape. Thus, it takes the shape of its container. A solid: has a fixed volume and a fixed shape. Note: changes in temperature or pressure causes the conversion from one physical state to another. In a gas, the molecules are far apart and moving at high speeds, colliding repeatedly with one another and with the walls of the container.
Get access
Grade+
$40 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
10 Verified Answers
Class+
$30 USD/m
Billed monthly

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
7 Verified Answers