CHEM 101 Lecture Notes - Lecture 8: Magnetic Quantum Number, Photographic Plate
CHEM 101 verified notes
8/40View all
Document Summary
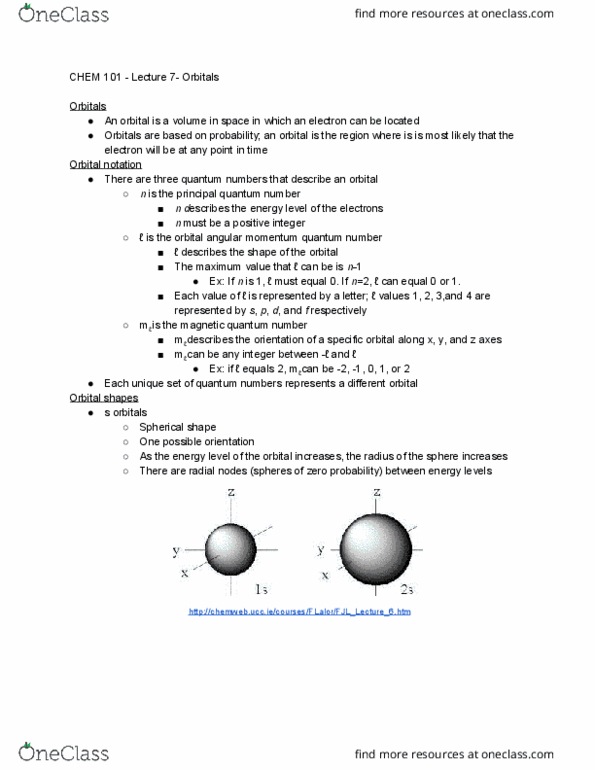
Orbitals represent the region with the greatest probability of finding an electron. The center of the orbital has a higher electron density than the edges. Orbitals are not walls ; there is a possibility of an electron being outside of its orbital. Orbitals are described by a principal quantum number (n), an orbital angular momentum quantum number ( ), and a magnetic quantum number (m ) Each orbital has a unique combination of these three quantum numbers. P orbitals ( =1, m = -1, 0, or 1) are dumbbell shaped. D orbitals ( =2, m = -2, -1, 0, 1, or 2) are mainly clover-leaf shaped. An energy level diagram shows the energy that each orbital has. Each row in the diagram is made up of orbitals in the same shell (orbitals that have the same principal quantum number) The groups within each level contain orbitals within one subshell (orbitals that have the same shape, but different orientations)