CHEM 1127Q Lecture Notes - Lecture 15: Equivalence Point, Thermite, Stoichiometry
CHEM 1127Q verified notes
15/27View all
Document Summary
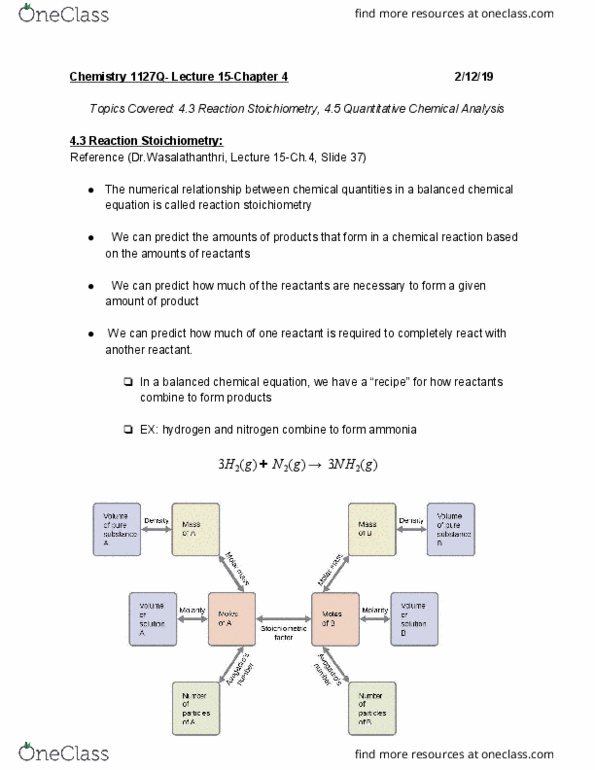
Topics covered: 4. 3 reaction stoichiometry, 4. 5 quantitative chemical analysis. The numerical relationship between chemical quantities in a balanced chemical equation is called reaction stoichiometry. We can predict the amounts of products that form in a chemical reaction based on the amounts of reactants. We can predict how much of the reactants are necessary to form a given amount of product. We can predict how much of one reactant is required to completely react with another reactant. In a balanced chemical equation, we have a recipe for how reactants combine to form products. Ex: hydrogen and nitrogen combine to form ammonia. 2 are required to react with 2. 50 mol of. Suppose we have 3 cups flour, 10 eggs, and 4 tsp baking powder. **the flour would be the limiting reagent; it is completely consumed and yields the least about of pancakes**