CHEM 07200 Midterm: Orgo lab 3

Denisse Cruz
09/26/16
Distillation
Performed on: 09/21/16
Purpose: The purpose of this lab was to perform a fractional distillation and examine how the
acetone condenses and evaporates mixed with water.
Lab Safety: This lab requires For this experiment, it was necessary to wear goggles, a lab coat,
and gloves. To ensure our safety for this lab we had to locate all exists in a case of an
emergency such as a fire. And we made sure to dispose of any organics in the waste solvent
bottle.
Materials and Equipment:
Heat mantle
Thermometer
Distillation Glassware
Boiling flask
Boiling stones
K clips
Stir-bar
Vacuum tubes
Grease
Collection flask
Condenser
Beads
Clamps
Procedure:
Part 1:
1. Measure 70 mL of the mixture into a 100mL round bottom flask and then add 5 boiling stones
to this mixture.
2. Use the round bottom flask in the fractional distillation setup. Make sure you apply a very
small amount of vacuum grease, as well as the blue Keck clips around the ground glass joints.
Use a 100mL graduated cylinder as the ڔcollection flask.ڕ
3. Record the temperature at every 2 mL of distillate, stop when all the low boiling liquid has
distilled (around 100 degrees C). Observe the increase in temperature to the boiling point of
water to ensure complete distillation of the lower boiling component of the mixture. Be sure to
STOP your distillation before it drys.
4. Pour the liquid distillate into the waste solvent bottle in the hood. Never pour organics down
the drain.
Part 2:
1. Measure 70 mL of the unknown mixture into a 100mL round bottom flask and then add a few
5 boiling stones to this mixture.
2. Use the above round bottom flask in the distillation setup. Make sure you apply a very small
amount of vacuum grease, as well as the blue Keck clips around the ground glass joints. Use a
100mL graduated cylinder as the ڔcollection flask.ڕ
3. Record the temperature at every 2 mL of distillate, stop when all the low boiling liquid has
distilled (around 100 degrees C). Observe the increase in temperature to the boiling point of
water to ensure complete distillation of the lower boiling component of the mixture. Be sure to
STOP your distillation before it drys.
4. Pour the liquid distillate into the waste solvent bottle in the hood. Never pour organics down
the drain.
5. For each distillation, graph temperature measurements on the Y axis and volume
distilled on X axis. Use excel to plot these graphs. Use the graph to estimate the boiling points
of the two components in the mixture. Be sure to specifically highlight and/or indicate somehow
find more resources at oneclass.com
find more resources at oneclass.com
the point on your graph that allows you to determine the boiling point for each component of the
mixture acetone and water.
Data:
Observations- Once everything was set up correctly we began our experiment. We observed
the liquid turned to gas while moving through the distillation and then would condense and fall
back down to the bottom with the boiling solution. When it made it up to the cooling part of the
distillation set up and the gas would condense into a liquid and pour out into the collection
beaker. We then performed the same procedure for the known mixture which was acetone. The
acetone started to boil at around 36mL. We noticed an increase in temperature which indicates
it was boiling water. For the unknown mixture we noticed around 25mL there was a peak
meaning the acetone was completely boiled over with an increase in temperature because it
was just boiling water.
Calculations-
Known mixture
The percentage of acetone in the known mixture was 50%.
35mL/70mL=50% acetone
Unknown mixture
The percentage of acetone in the unknown mixture was 35.7%
25mL/70mL=35.7% acetone.
Results:
We also had to calculate the percentage of acetone in the known and unknown mixture. We
concluded the amount of acetone that was in the known mixture was 50%. Because we
observed a high peak in temperature around 35 mL, that means all the acetone was fully boiled
out of the mixture and began to boil the water. Therefore, we took the 35 mL and divided that by
the amount of the initial mixture (70)mL.
35mL/70mL=50%
For the unknown mixture, the acetone was boiling out of the mixture around 25 mL which
indicates there would be less acetone in this mixture. Therefore, we divided 25 by the amount of
the initial mixture (70)mL.
25mL/70mL=37.7%.
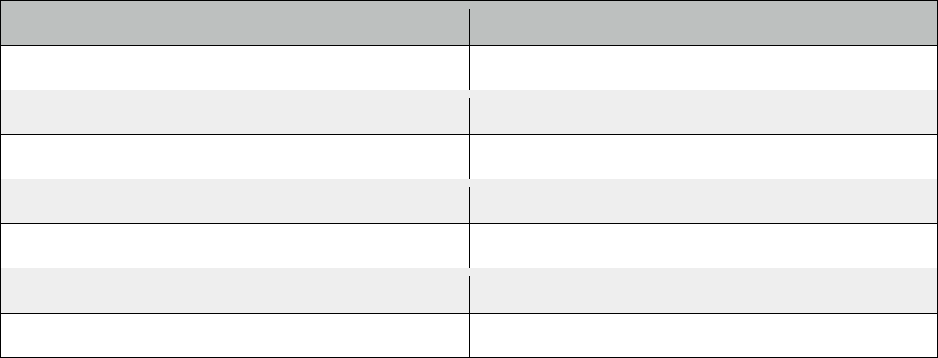
Known Mixture
mL
Temperature (degrees Celsius)
2
57
4
58
6
58
8
58
10
58
12
58
14
58
find more resources at oneclass.com
find more resources at oneclass.com
Document Summary
Purpose: the purpose of this lab was to perform a fractional distillation and examine how the acetone condenses and evaporates mixed with water. Lab safety: this lab requires for this experiment, it was necessary to wear goggles, a lab coat, and gloves. To ensure our safety for this lab we had to locate all exists in a case of an emergency such as a fire. And we made sure to dispose of any organics in the waste solvent bottle. Part 1: measure 70 ml of the mixture into a 100ml round bottom flask and then add 5 boiling stones to this mixture, use the round bottom flask in the fractional distillation setup. Use a 100ml graduated cylinder as the (cid:1684)collection flask. (cid:1685: record the temperature at every 2 ml of distillate, stop when all the low boiling liquid has distilled (around 100 degrees c).








