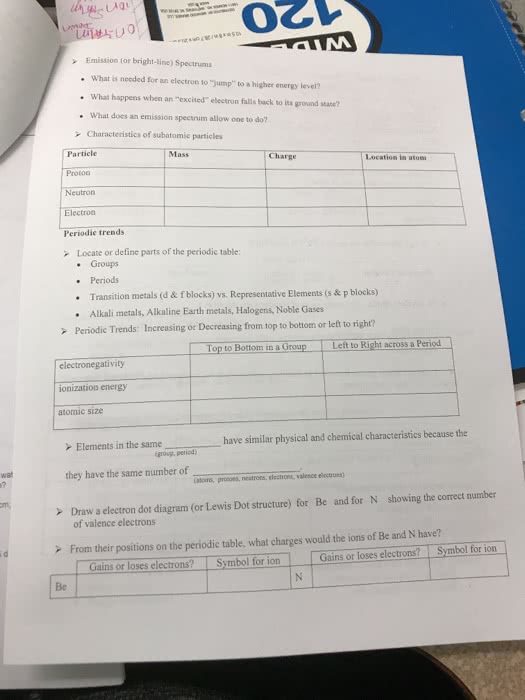
Decomposition of Malachite
Here is what Malachite is Made Out Of
Malachite is a famous and very popular semi-precious stone. Its banded light and dark green bands are one-of-a-kind, and give it a unique ornamental quality unlike that of any other stone. The light and dark green bands are so distinctive that malachite may be one of the most easily recognized minerals by the general public. Malachite is popular in jewelry, especially Native American southwestern jewelry. The stones inlayed in silver make a nice alternative to traditional turquoise jewelry.
Malachite is a form of copper carbonate with the molecular formula Cu2CO3(OH)2. When heated to around 200 �C, malachite decomposes into copper (II) oxide (CuO) and other products.
Reaction Stoichiometry
The Law of Conservation of Mass states that matter can neither be created nor destroyed. Therefore, the number of atoms of each element on the reactants side must be same as the number of atoms on the products side. This quantitative study of chemical reactions is called stoichiometry. The substances in a balanced reaction are related to each other by stoichiometric molar ratios. For example, magnesium reacts with hydrochloric acid in a 1:2 stoichiometric ratio in the balanced equation below.
Mg(s) + 2HCl(aq) -> MgCl2(aq) + H2(g)
The molar ratios can be used as conversion factors to find the amount (in moles) of one substance given the amount of another. For example, given the number of moles of HCl, the amount of MgCl2 can be determined as shown below.
nMgCl2=nHCl x (1mol MgCl2) / (2mol HCL)
The molar ratios are used to determine the amount in moles. However, the amount of reactant or product is often measured in grams. Mass can be converted to moles and vice versa as shown below.
n = m / (MM)
m = n x MM
where n is the number of moles, m is the mass of the product in g, and MM is its molar mass in g/mol.
To observe the Law of Conservation of Mass, you can perform different types of chemical reactions. For example, you can use a decomposition reaction to observe one compound being broken down into two or more elements or new compounds. For this reaction to take place, you must break existing bonds within the starting compound. Different sources of energy such as heat, light, or electricity may be used. Through a decomposition reaction, you can observe that the atoms in the original compound are not destroyed but are rearranged to form simpler substances, such as elements or compounds.
About This Lab
In this lab, you will test if the relationship between malachite and copper (II) oxide is stoichiometric. To do this, you will heat a known mass of malachite to produce copper (II) oxide. You will then compare the amount of copper (II) oxide obtained experimentally with the amount of copper (II) oxide expected from the balanced equation for the decomposition reaction.
1.Take a crucible from the Containers shelf and place it on the workbench.
2.Take a balance from the Instruments shelf and place it on the workbench.
3.Move the crucible onto the balance. Record the mass of the empty crucible to the nearest 0.001g in your Lab Notes.
4.Move the crucible onto the workbench.
5.Take copper (II) carbonate hydroxide from the Materials shelf and add 10 g to the crucible.
6.Move the crucible onto the balance and record the mass of the crucible containing copper (II) carbonate hydroxide in your Lab Notes.
7.Take a Bunsen burner from the Instruments shelf and place it on the workbench.
8.Move the crucible onto the Bunsen burner.
9.Double-click on the crucible to open its properties. Select Show Contents and click OK. This gives you a cut-away view of the crucible's contents.
10.Turn the Bunsen burner on to low flame by clicking the knob at the bottom once. Clicking multiple times will increase the intensity of the flame until it is turned off.
11.Watch the crucible until the malachite fully decomposes. You will see a distinct color change.
12.Move the crucible onto the workbench to cool.
13.Turn off your Bunsen burner.
14.Weigh the crucible with its contents and record the mass to the nearest 0.001 g in your Lab Notes.
Clear your station.
Lab Notes:
Numbers below refer to numbers in the above âAbout this Labâ
3. Empty crucible = 88 g
5. Added 10 g of Copper (II) carbonate hydroxide to crucible
6. Crucible with Copper (II) carbonate hydroxide = 98 g
11. to Black
14. after heating crucible with its contents = 95.195
Questions I have answered:
How many grams of malachite did you add to the crucible? 10g
How many grams of product were in the crucible after heating? 7.195
By how much did the mass of the crucible's contents change after heating? -2.805
The molar mass of malachite is 221.1 g/mol. How many moles of malachite were present before the reaction? 0.04523 mol
The product remaining in the crucible after heating is copper (II) oxide. The molar mass of copper (II) oxide is 79.55 g/mol. How many moles of copper (II) oxide were produced during the reaction?
0.0945 mol
Please answer the following:
Q1 a. Which of the following represents the balanced chemical equation for this reaction?
Cu2CO3(OH)2=2CuO+H2O
Cu2CO3(OH)2=CuO+CO2
Cu2CO3(OH)2=2CuO+H2O+CO2
Cu2CO3(OH)2=CuO+H2O+CO2
b. Based on the number of moles of malachite that you started with, how many grams of water were produced? The molar mass of water is 18.0153 g/mol. Choose the closest answer.
1.815 g 0.8148 g 1.630 g 1.982 g
c. Based on the number of moles of malachite that you started with, how many moles of CO2were produced? Choose the closest answer.
1.000 mol 0.04523 mol 0.9000 mol 0.09045 mol
d. Based on the number of moles of malachite that you started with, how many grams of CO2were produced? The molar mass of CO2 is 44.01 g/mol. Choose the closest answer.
1.991 g 0.4401 g 0.04523 g 3.981 g
Q2a. Based on the number of grams of CO2 and H2O produced during the reaction, does this compensate for the observed loss of mass?
A. No, because this violates the law of conservation of mass
B. yes, this compensates for the mass lost in the reaction
C. No, because more copper oxide was produced
D. No because mass is conserved
b. You observed that the products in the crucible weigh less than the reactants that you added. How is this possible?
A. Mass was lost due to experimental error
B. The Malachite absorbed CO2 from the air
C. CO2 and H2O were released as gases
D. Mass was converted to energy
c. What kind of a reaction took place upon heating?
A. decomposition
B. single displacement
C. synthesis
D. Double displacement
d. Baking soda is used as a leavening agent in baking. When heated, baking soda undergoes a decomposition reaction to form carbon dioxide. Given the data below, which of the following reactions occurs during baking? The molar mass of baking soda is 84.007 g/mol and the molar mass of carbon dioxide is 44.01 g/mol.
Mass of empty crucible (g) = 55.000
mass of crucible with baking soda (g) = 57.000
mass of crucible and contents after the reaction (g) =56.260
A. 2NaHCO3= 2Na+2CO2 +H2O
B. 2NaHCO3 = Na2O + 2CO2 +H2O
C. NaHCO3 = NaOH +CO2
D. 2NaHCO3 = Na2CO3 + CO2 +H2O