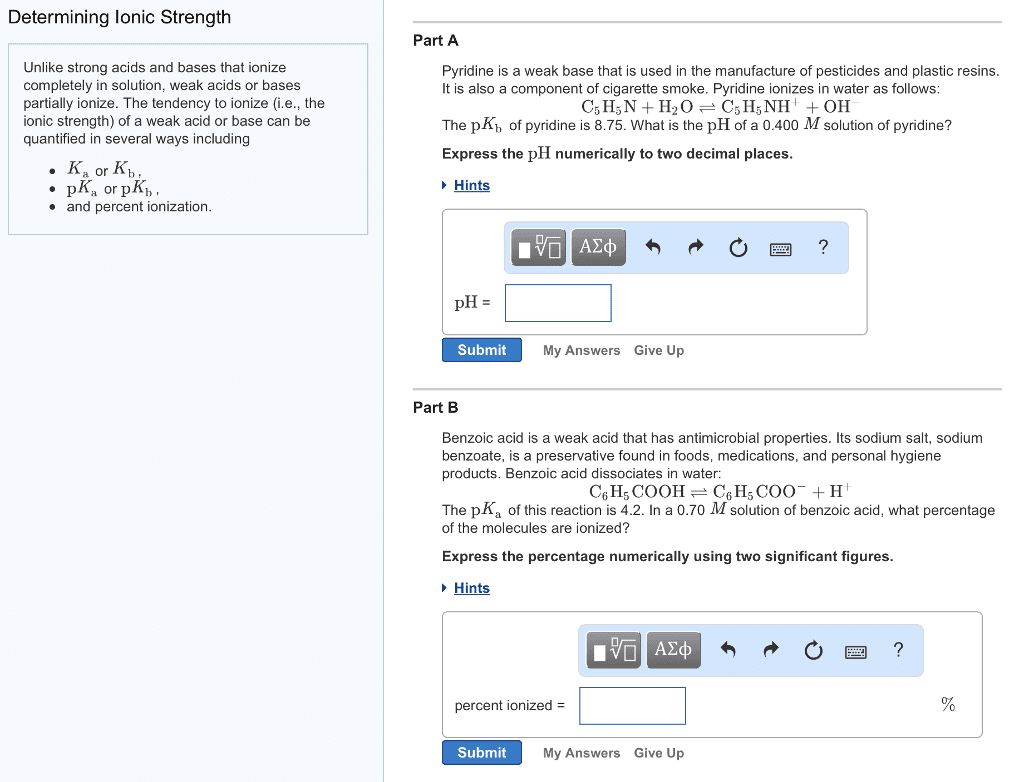
CHEM 142 Study Guide - Midterm Guide: Conjugate Acid, Litmus, Carboxylic Acid
Document Summary
Alkaloid: organic bases found in plants that are often poisonous; our aversion to the taste of bases is probably an evolutionary adaptation to warn us against these. Arrhenius definitions (of acids and bases): says an acid is a substance that produces h+ ions in aqueous solution; a base is a substance that produces oh ions in aqueous solution. Strong acid: acid that completely ionizes in solution. Weak acid: acid that only partially ionizes in solution. Strong base: base that completely dissociates in solution. Weak base: base that partially dissociates in solution. Br nsted lowry definitions (of acids and bases): says an acid is a proton (h+) donor and becomes a conjugate base; a base is a proton (h+) acceptor and becomes a conjugate acid. Conjugate acid: any base to which a proton has been added. Conjugate base: any acid from which a proton has been removed. Monoprotic acid: acids containing only one ionizable proton.