1
answer
0
watching
124
views
6 Oct 2020
Textbook Problem
A sample of an ideal gas goes through the process shown in Figure P20.34. From A to B. The process is adiabatic; from B to C, it is isobaric with 345 kJ of energy entering the system by heat: from C to D, the process is isothermal; and from D to A, it is isobaric with 371 kJ of energy leaving the system by heat. Determine the difference in internal energy Eint.B – Eint.A.
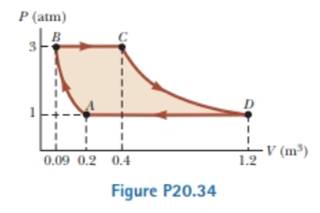
Textbook Problem
A sample of an ideal gas goes through the process shown in Figure P20.34. From A to B. The process is adiabatic; from B to C, it is isobaric with 345 kJ of energy entering the system by heat: from C to D, the process is isothermal; and from D to A, it is isobaric with 371 kJ of energy leaving the system by heat. Determine the difference in internal energy Eint.B – Eint.A.

PriyankaLv10
26 Nov 2020



