1
answer
0
watching
236
views
7 Apr 2019
286 mols of an ideal monatomic gas undergo the process shown in the PV plot (Figure 1). The process A→B is adiabatic. In the plot below, Volume A=1.60 m3, Volume B=3.48 m3 and Pressure A=3.14 atm.
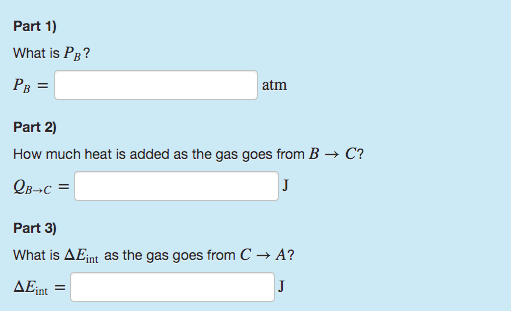
What is Pressure B?
How much heat is added as the gas goes from B→C?
What is ΔEint as the gas goes from C→A?
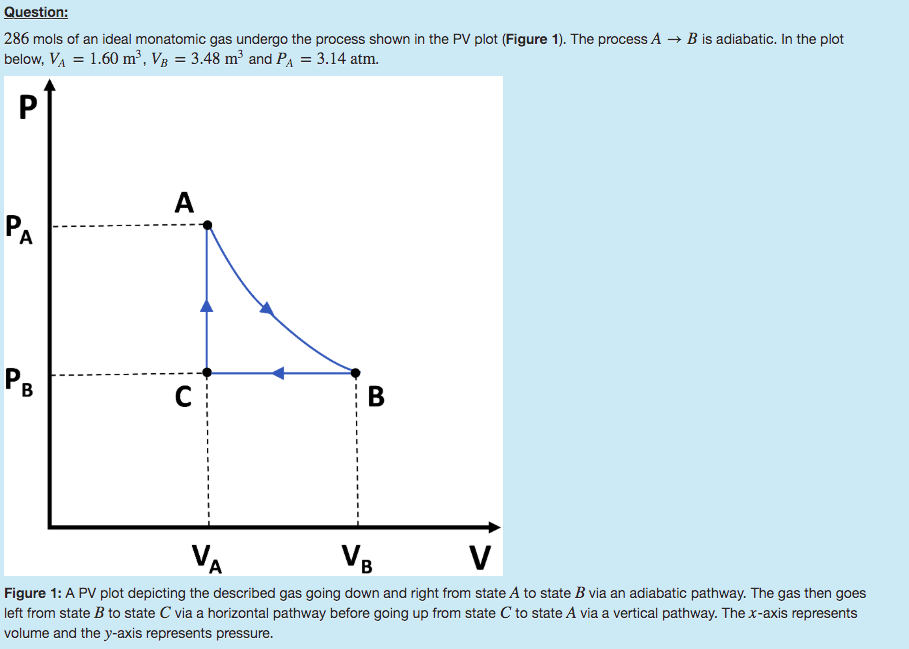
286 mols of an ideal monatomic gas undergo the process shown in the PV plot (Figure 1). The process A→B is adiabatic. In the plot below, Volume A=1.60 m3, Volume B=3.48 m3 and Pressure A=3.14 atm.
What is Pressure B?
How much heat is added as the gas goes from B→C?
What is ΔEint as the gas goes from C→A?


Tod ThielLv2
17 Feb 2021
