1
answer
2
watching
882
views
6 Apr 2020
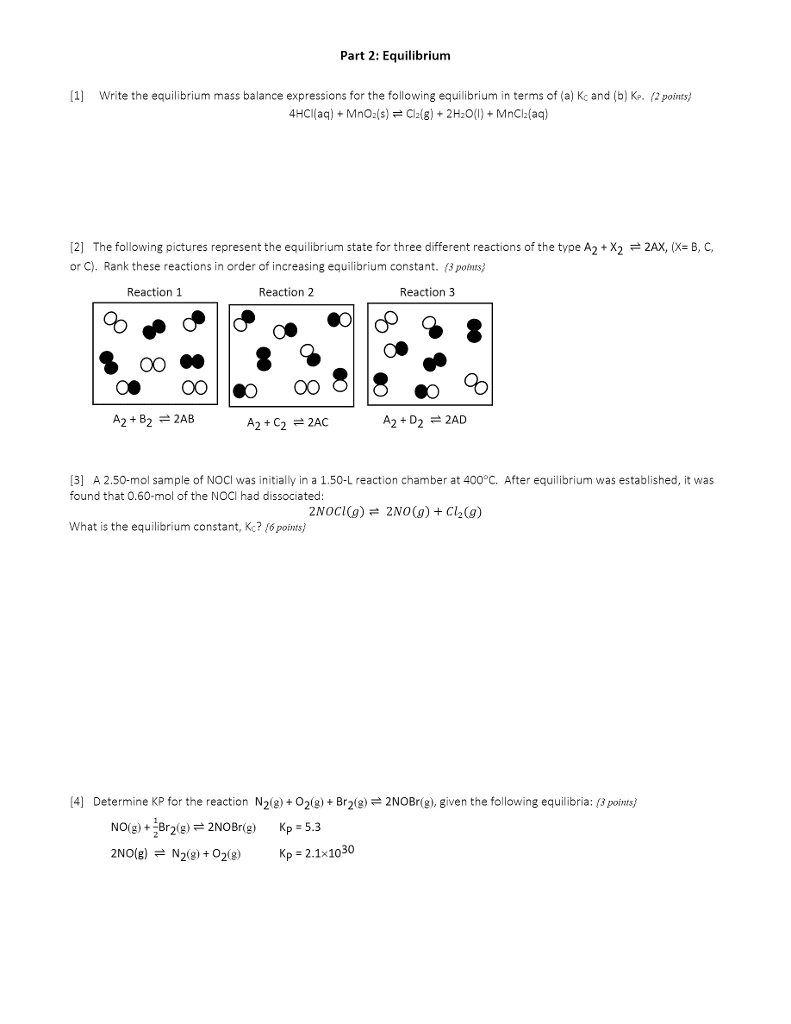
The reaction A2 + B2  2 AB has an equilibrium constant Kc = 1.5. The following diagrams represent reaction mixtures containing A2 molecules (red), B2 molecules (blue), and AB molecules. (a) Which reaction mixture is at equilibrium? (b) For those mixtures that are not at equilibrium, how will the reaction proceed to reach equilibrium?
2 AB has an equilibrium constant Kc = 1.5. The following diagrams represent reaction mixtures containing A2 molecules (red), B2 molecules (blue), and AB molecules. (a) Which reaction mixture is at equilibrium? (b) For those mixtures that are not at equilibrium, how will the reaction proceed to reach equilibrium?

The reaction A2 + B2 2 AB has an equilibrium constant Kc = 1.5. The following diagrams represent reaction mixtures containing A2 molecules (red), B2 molecules (blue), and AB molecules. (a) Which reaction mixture is at equilibrium? (b) For those mixtures that are not at equilibrium, how will the reaction proceed to reach equilibrium?
Keith LeannonLv2
23 May 2020