1
answer
0
watching
236
views
28 Sep 2019
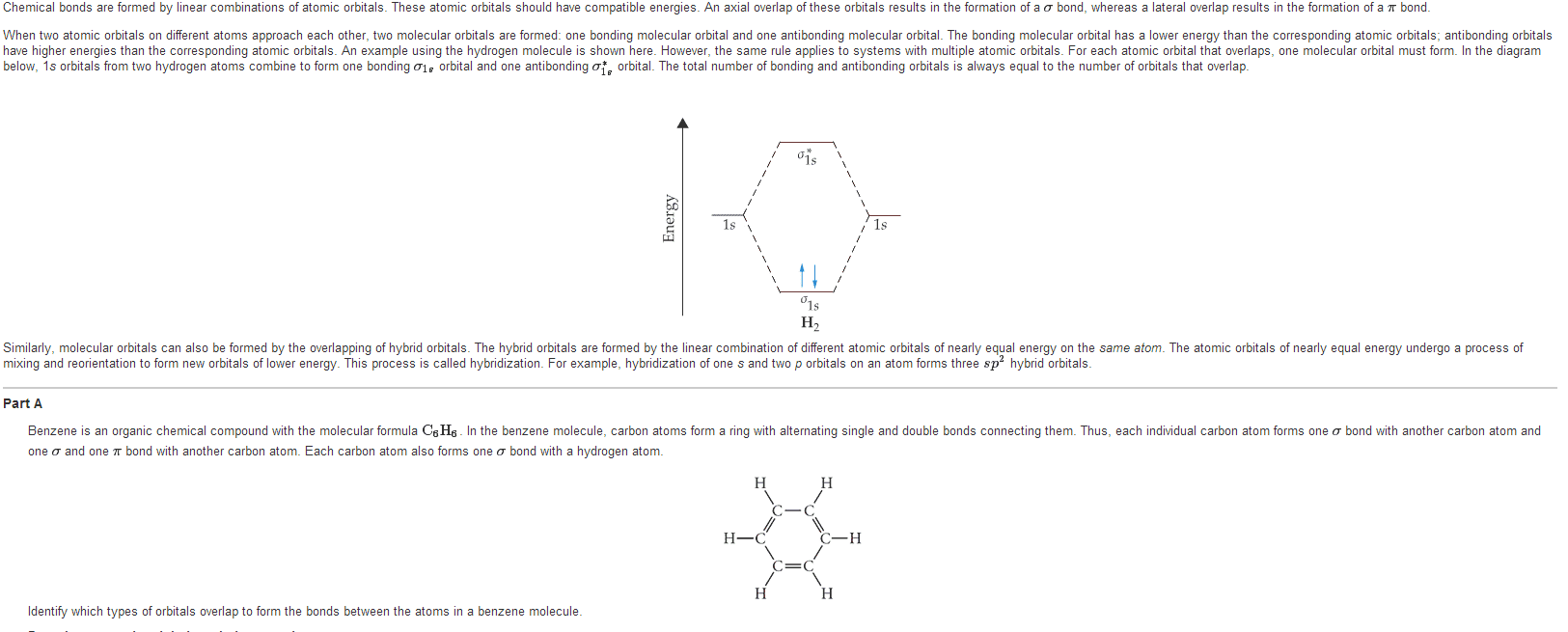
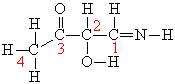
Which statement correctly correlates s or p bonding atoms and theirrespective bonding orbitals in formaldehyde?
A)The oxygen atom (with its 2s orbital) forms a s bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom.
B)The oxygen atom (with its 2p orbital) forms a p bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom.
C)The oxygen atom (with its 2p orbital) forms a p bond with the pzorbital of the carbon atom; the two hydrogen atoms (with their 1sorbitals) each form a s bond with one of the two sp2 hybridorbitals of the carbon atom.
D)The oxygen atom (with its 2p orbital) forms a s bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom. The oxygen atom alsoforms a p bond with carbon which involves the 2pz orbitals ofoxygen and carbon.
Which statement correctly correlates s or p bonding atoms and theirrespective bonding orbitals in formaldehyde?
A)The oxygen atom (with its 2s orbital) forms a s bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom.
B)The oxygen atom (with its 2p orbital) forms a p bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom.
C)The oxygen atom (with its 2p orbital) forms a p bond with the pzorbital of the carbon atom; the two hydrogen atoms (with their 1sorbitals) each form a s bond with one of the two sp2 hybridorbitals of the carbon atom.
D)The oxygen atom (with its 2p orbital) forms a s bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom. The oxygen atom alsoforms a p bond with carbon which involves the 2pz orbitals ofoxygen and carbon.
A)The oxygen atom (with its 2s orbital) forms a s bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom.
B)The oxygen atom (with its 2p orbital) forms a p bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom.
C)The oxygen atom (with its 2p orbital) forms a p bond with the pzorbital of the carbon atom; the two hydrogen atoms (with their 1sorbitals) each form a s bond with one of the two sp2 hybridorbitals of the carbon atom.
D)The oxygen atom (with its 2p orbital) forms a s bond with one ofthe three sp2 hybrid orbitals of the carbon atom; the two hydrogenatoms (with their 1s orbitals) each form a s bond with one of thethree sp2 hybrid orbitals of the carbon atom. The oxygen atom alsoforms a p bond with carbon which involves the 2pz orbitals ofoxygen and carbon.
Casey DurganLv2
28 Sep 2019