1
answer
0
watching
710
views
6 Nov 2019
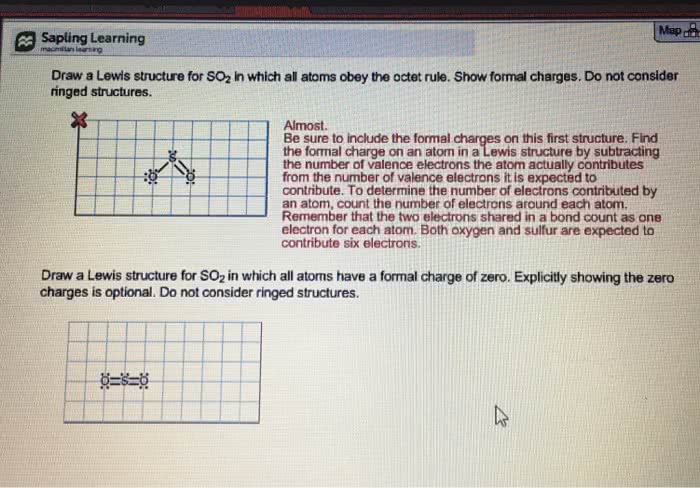
Draw Lewis structures of the four molecules and/or ions with the formulas given below and figure out which two of these are isoelectronic with each another. Species which are isoelectronic have the same number of atoms, the same total number of electrons, the same number of valence electrons, and the same valence electron structure (single and multiple bonds and lone pairs in same places), but need not have the same distribution of formal charges on atoms. CO2 and ClO2 and NO2+ and SO2
Draw Lewis structures of the four molecules and/or ions with the formulas given below and figure out which two of these are isoelectronic with each another. Species which are isoelectronic have the same number of atoms, the same total number of electrons, the same number of valence electrons, and the same valence electron structure (single and multiple bonds and lone pairs in same places), but need not have the same distribution of formal charges on atoms. CO2 and ClO2 and NO2+ and SO2
Patrina SchowalterLv2
6 Nov 2019