CHEM 1127Q Lecture Notes - Lecture 6: Ionic Bonding, Nonmetal, Sodium Nitrate
CHEM 1127Q verified notes
6/27View all
Document Summary
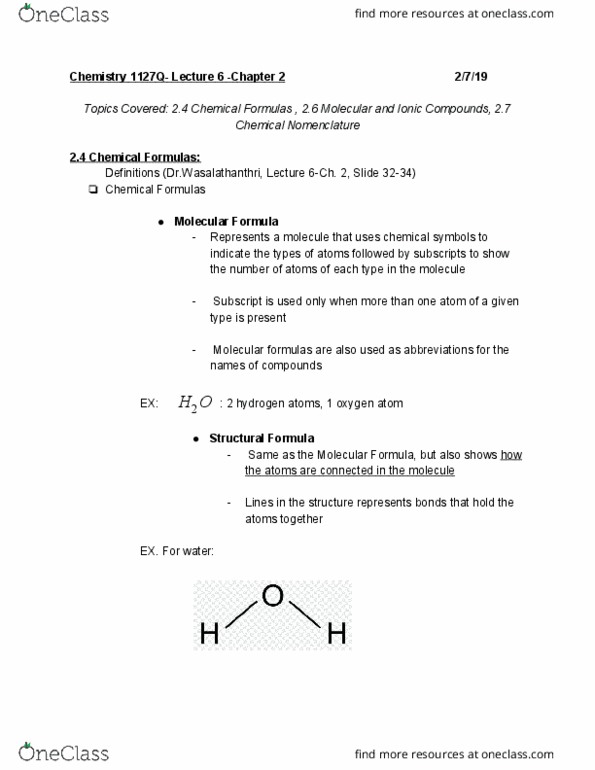
Topics covered: 2. 4 chemical formulas , 2. 6 molecular and ionic compounds, 2. 7. Represents a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule. Subscript is used only when more than one atom of a given type is present. Molecular formulas are also used as abbreviations for the names of compounds. Same as the molecular formula, but also shows how the atoms are connected in the molecule. Lines in the structure represents bonds that hold the atoms together. Indicates the simplest whole number ratio of the number of atoms (or ions) in the compound. Compounds made up of ions held together by electrostatic forces (ionic bonding ) By removing electrons from a neutral atom cations are produced. By adding electrons from a neutral atom anions are produced. You should be familiar with polyatomic ions. Electrons are shared between atoms in these compounds.