ENGR 1A Lecture Notes - Lecture 27: Lattice Energy, Ionic Compound
ENGR 1A verified notes
27/31View all
Document Summary
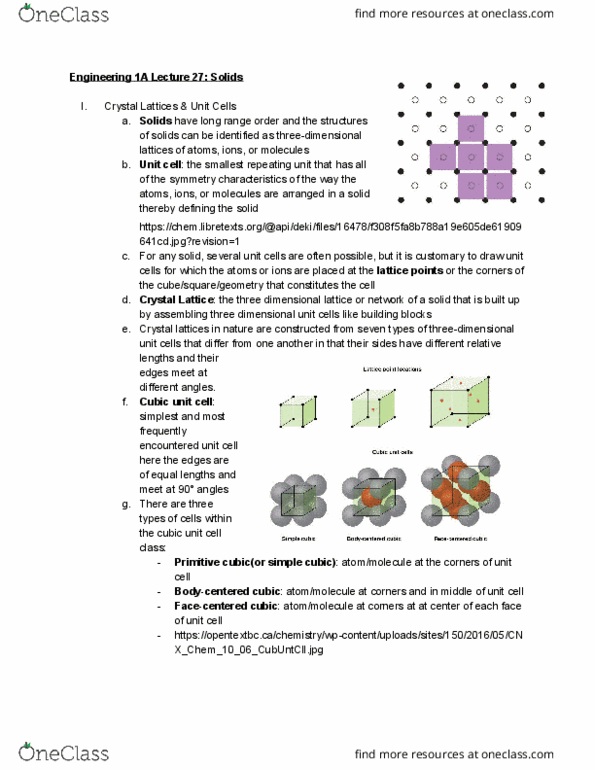
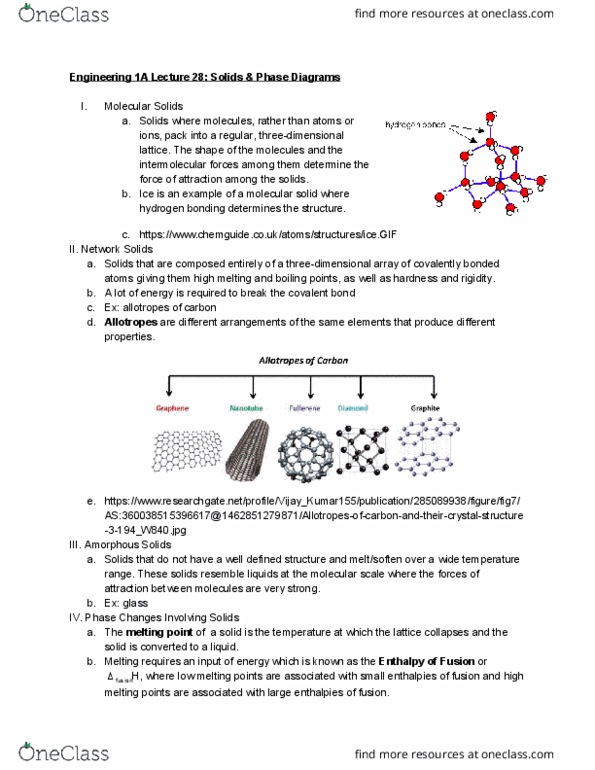
Have long range order and the structures of solids can be identified as three-dimensional lattices of atoms, ions, or molecules: unit cell . : the smallest repeating unit that has all of the symmetry characteristics of the way the atoms, ions, or molecules are arranged in a solid thereby defining the solid https://chem. libretexts. org/@api/deki/files/16478/f308f5fa8b788a19e605de61909. 641cd. jpg?revision=1 cells for which the atoms or ions are placed at the lattice points the cube/square/geometry that constitutes the cell: for any solid, several unit cells are often possible, but it is customary to draw unit. : atom/molecule at corners and in middle of unit cell. : atom/molecule at corners at at center of each face of unit cell https://opentextbc. ca/chemistry/wp-content/uploads/sites/150/2016/05/cn. Aluminum has a density of 2. 699 g/cm 3 , and the atoms are packed in a face-centered. Structure and formulas of ionic solids: octahedral holes , tetrahedral holes a. : each ion is surrounded by six oppositely charged ions.