CHEM 1210 Lecture Notes - Lecture 20: Planck Constant, Photon, Ultraviolet Catastrophe
CHEM 1210 verified notes
20/46View all
Document Summary
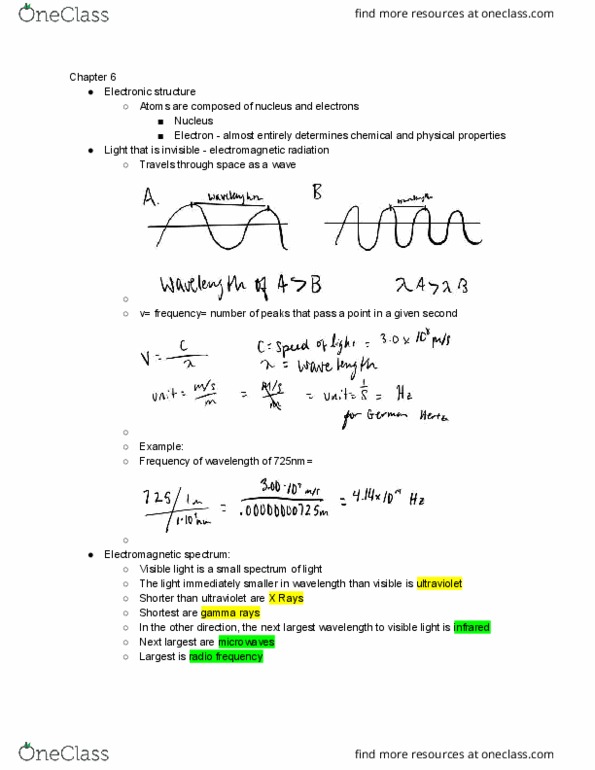
Atoms are composed of nucleus and electrons. Electron - almost entirely determines chemical and physical properties. Light that is invisible - electromagnetic radiation. V= frequency= number of peaks that pass a point in a given second. Visible light is a small spectrum of light. The light immediately smaller in wavelength than visible is ultraviolet. In the other direction, the next largest wavelength to visible light is infrared. The whole spectrum from shortest to longest wavelength goes: Gamma rays, x rays, ultraviolet , visible light, infrared, microwaves, radio frequency. A higher frequency means shorter wavelengths and higher energy. All wavelengths travel at the same speed: 3. 00 x 10 8. Compared to light, sound waves travel slowly. High temperature leads to shorter frequency with higher energy. This was impossible to explain with classic thermodynamics. Nobody could explain the black body radiation dip down at the beginning of the curve, it would be expected to go up, according to thermodynamics.