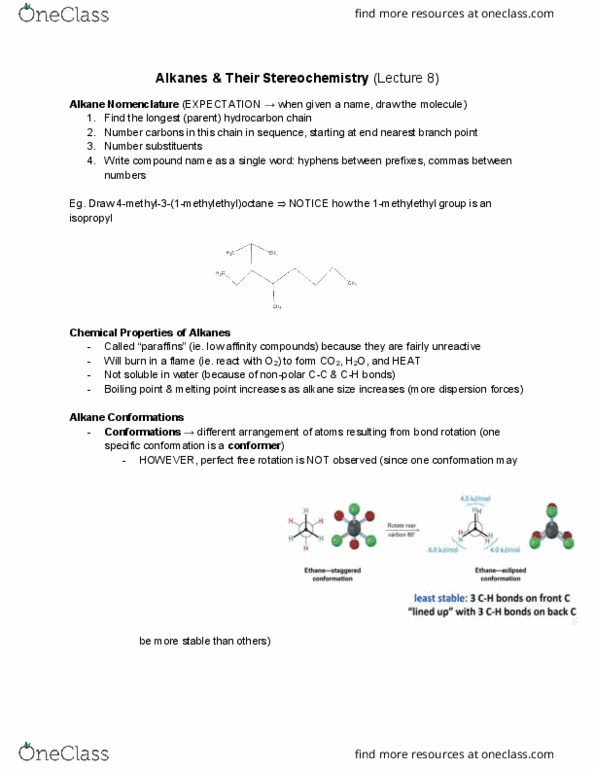
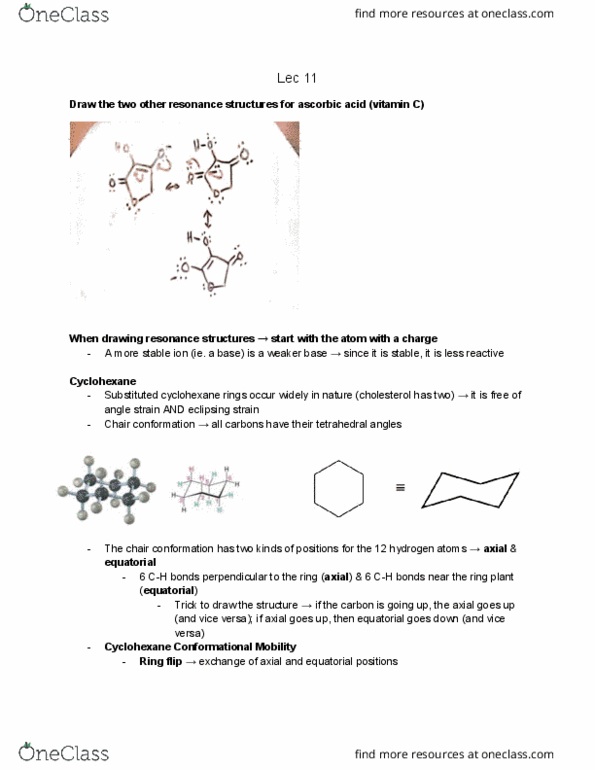
CHM136H1 Lecture Notes - Lecture 10: Tetrahedron, Ring Strain, Cycloalkane
CHM136H1 verified notes
10/39View all
Document Summary
Cyclic compounds commonly encountered in all classes of biomolecules: Much less conformational freedom since the bonds cannot rotate. Stereoisomers same connections but different 3d geometry. Compare to constitutional isomers where there are different connections between atoms. Example to the left all of the bonds are the same, but the methyl groups are either on the same side or different sides (called diastereomers cannot interconvert through bond rotation) ** just like constitutional isomers, stereoisomers also have different physical properties ** Rings larger than three atoms are not flat. Adopt non-planar conformations to minimize angle strain (ie. anything that is away from the ideal angle; in this case, the tetrahedral angle, 109. 5) and eclipsing (torsional) strain. Larger rings are harder to analyze because they have many possible conformations. Angle strain expansion or compression of bond angles away from most stable. Eclipsing (torsional) strain eclipsing or alignment of bonds on neighbouring atoms. Steric strain repulsive interactions between non-bonded atoms in close proximity.