CHM136H1 Lecture 2: Intro to Organic Chemistry: January 9th
CHM136H1 verified notes
2/39View all
Document Summary
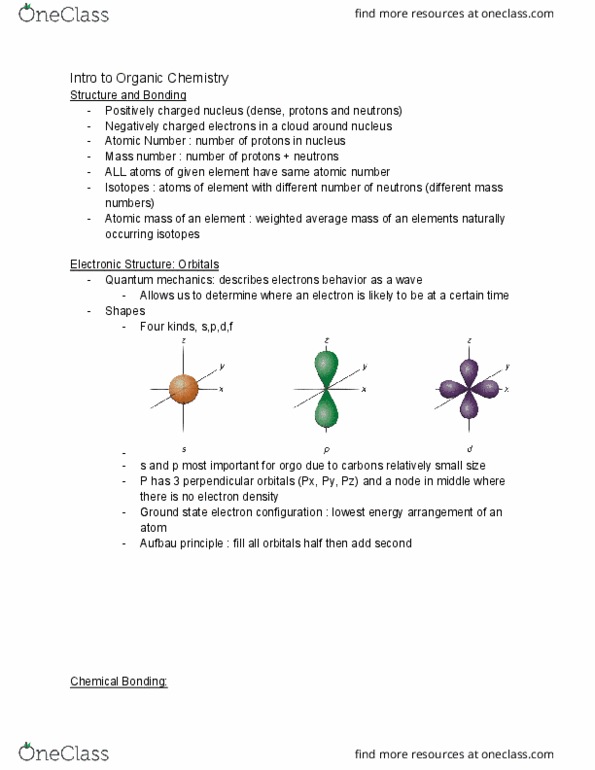
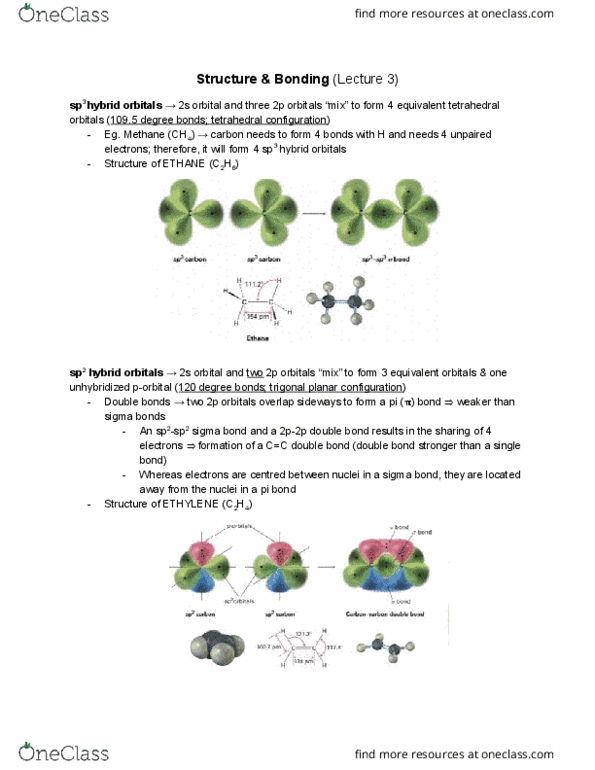
Positively charged nucleus (dense, protons and neutrons) Negatively charged electrons in a cloud around nucleus. Atomic number : number of protons in nucleus. Mass number : number of protons + neutrons. All atoms of given element have same atomic number. Isotopes : atoms of element with different number of neutrons (different mass numbers) Atomic mass of an element : weighted average mass of an elements naturally occurring isotopes. Quantum mechanics: describes electrons behavior as a wave. Allows us to determine where an electron is likely to be at a certain time. S and p most important for orgo due to carbons relatively small size. P has 3 perpendicular orbitals (px, py, pz) and a node in middle where there is no electron density. Ground state electron configuration : lowest energy arrangement of an atom. Aufbau principle : fill all orbitals half then add second. Electron goes from low ionization energy to high electron affinity.