CHM135H1 Lecture Notes - Lecture 5: Mnemonic, Quantum Number, Atomic Number
CHM135H1 verified notes
5/38View all
Document Summary
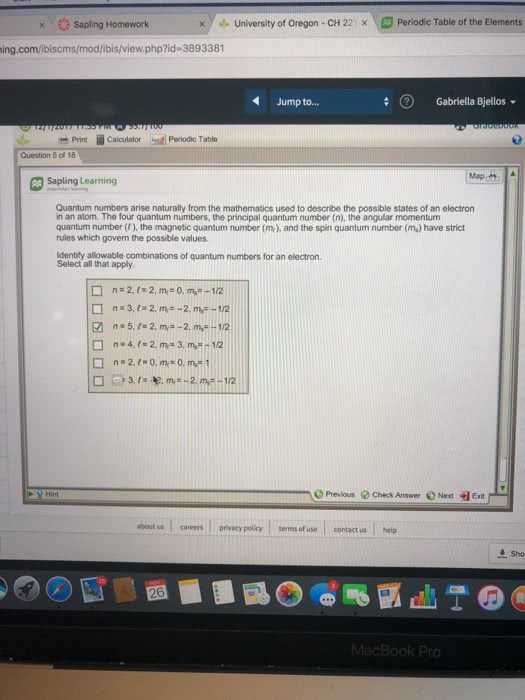
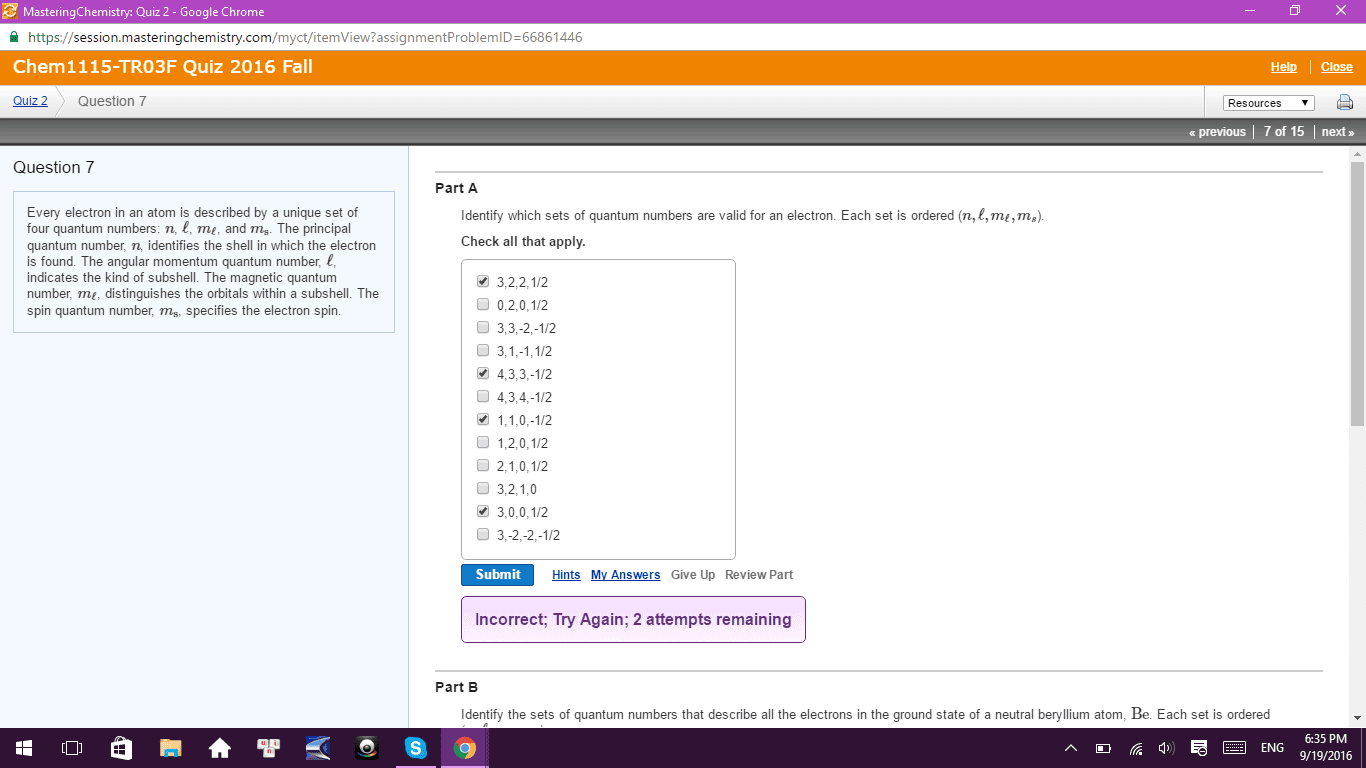
Orbitals using hydrogen functions but works for multi e systems. Exclusion no two e s can have the same 4 quantum. 2 angular momentum quantum number l f 0,1 shape of orbital n l. Ms each orbital can hold 2 e s. Goto f n l mi l to w e. 2 electron electron nucleus electron by attractions repulsions slide 1 p271. Slide ip 28 degeneracy configurato lowest e levels. 2es one orbital wore e goes into eoen degenerate orbitals until half full are available slide 1 p o mnemonic diagram of fitting orbitals much easier follow perodie table to e. g. S in ground state e s atomic number. Increases down a period left right across effective nuclear charge. Es held tightly up down a family more more e s orbitals e s are less farther away electrostatic attraction to nucleus.