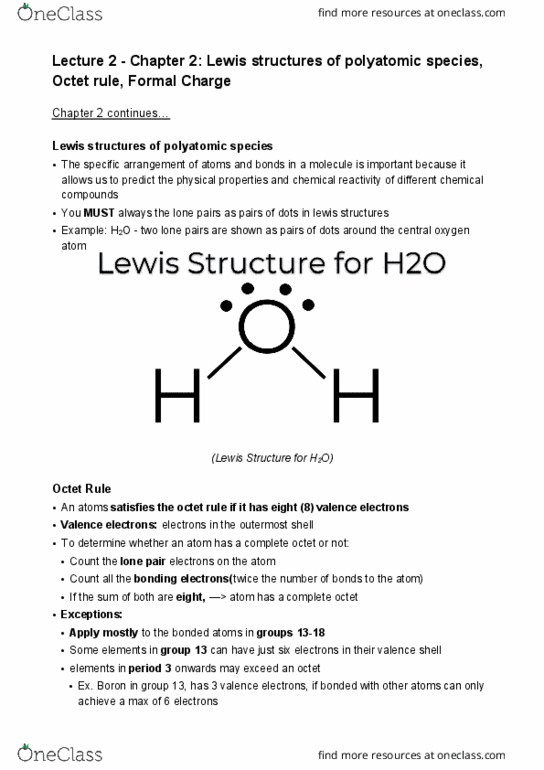
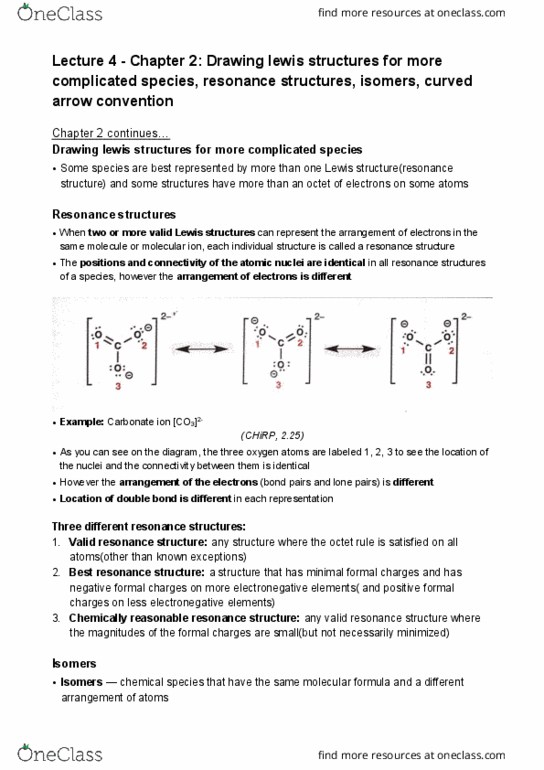
CHEM 121 Lecture Notes - Lecture 3: Lewis Structure, Formal Charge, Noble Gas
CHEM 121 verified notes
3/38View all
Document Summary
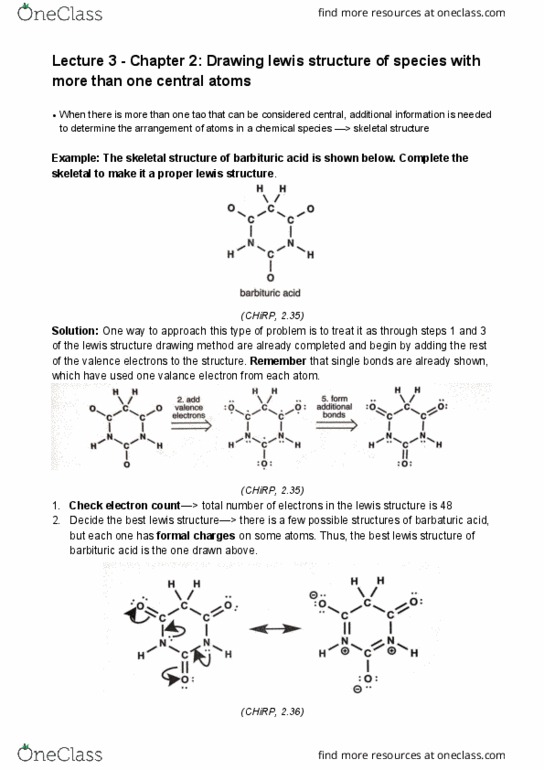
Example: the skeletal structure of barbituric acid is shown below. Complete the skeletal to make it a proper lewis structure. (chirp, 2. 35) Thus, the best lewis structure of barbituric acid is the one drawn above. (chirp, 2. 36) The number of valence electrons for all the atoms in the species, taking into account overall charge, must be shown as lines or dots in the lewis structure. When atoms bond to form molecules or ions, the total number of electrons conserved. Valence electrons are involved in bonding and in chemical reactions, therefore they are the only ones shown in the lewis structure. All electrons are assigned, either as bond pairs or line pairs on each atom in a species. This is the consequence of quantum mechanics, which will be discussed in chapters 4-5. In a lewis structure, atoms typically are surrounded by a octet of electrons.