CHEM103 Lecture Notes - Lecture 2: Ion, Electromagnetic Spectrum, Electric Field
CHEM103 verified notes
2/10View all
Document Summary
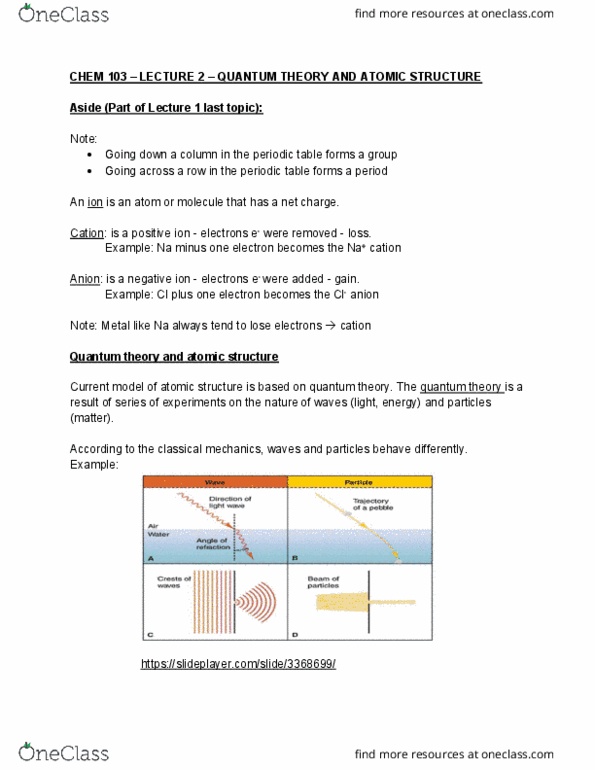
Chem 103 lecture 2 quantum theory and atomic structure. Note: going down a column in the periodic table forms a group, going across a row in the periodic table forms a period. An ion is an atom or molecule that has a net charge. Cation: is a positive ion - electrons e- were removed - loss. Example: na minus one electron becomes the na+ cation. Example: cl plus one electron becomes the cl- anion. Anion: is a negative ion - electrons e- were added - gain. Note: metal like na always tend to lose electrons cation. Current model of atomic structure is based on quantum theory. The quantum theory is a result of series of experiments on the nature of waves (light, energy) and particles (matter). According to the classical mechanics, waves and particles behave differently. Amplitude: is a measure of the displacement of the wave from its rest position https://slideplayer. com/slide/3368699/