CHEM101 Lecture Notes - Lecture 6: Atomic Orbital, Azimuthal Quantum Number
CHEM101 verified notes
6/41View all
Document Summary
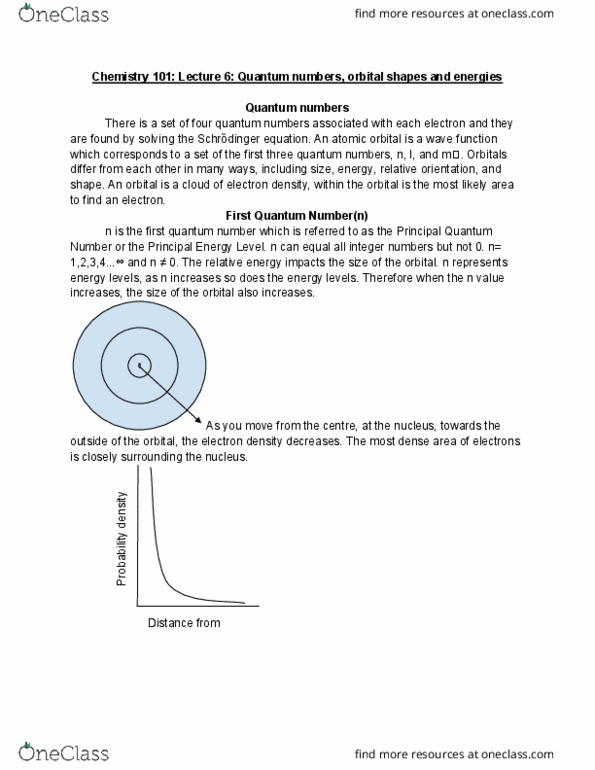
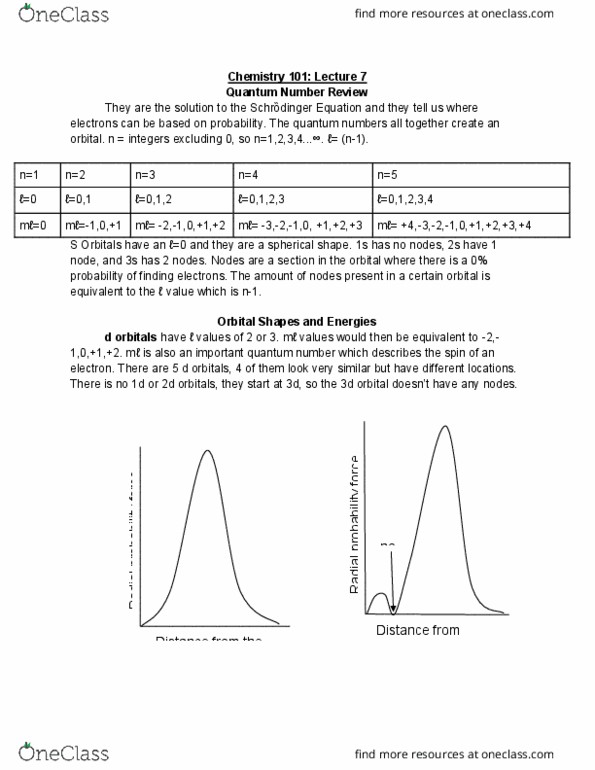
Chemistry 101: lecture 6: quantum numbers, orbital shapes and energies. There is a set of four quantum numbers associated with each electron and they are found by solving the schr dinger equation. An atomic orbital is a wave function which corresponds to a set of the first three quantum numbers, n, l, and m . Orbitals differ from each other in many ways, including size, energy, relative orientation, and shape. An orbital is a cloud of electron density, within the orbital is the most likely area to find an electron. First quantum number(n) n is the first quantum number which is referred to as the principal quantum. Number or the principal energy level. n can equal all integer numbers but not 0. n= The relative energy impacts the size of the orbital. n represents energy levels, as n increases so does the energy levels.