CHEM 1A Lecture Notes - Lecture 31: Bond Order, Lewis Structure, Zinc Sulfide
CHEM 1A verified notes
31/31View all
Document Summary
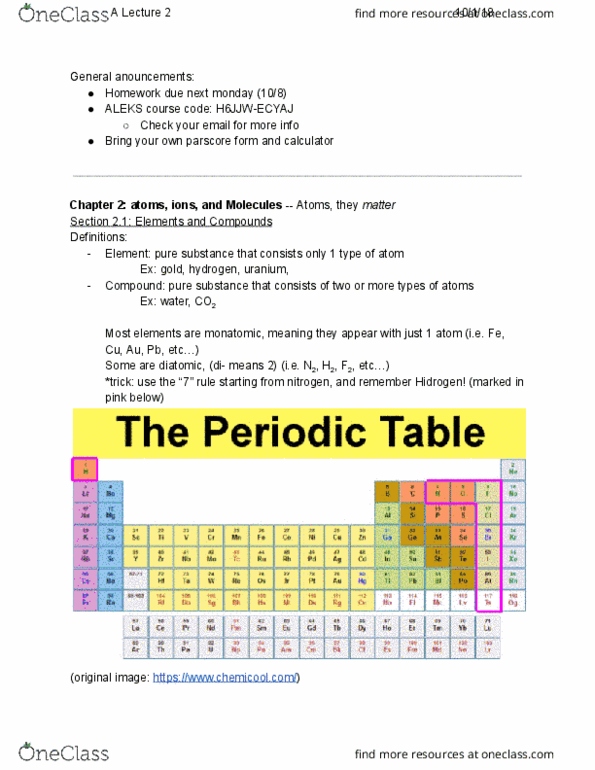
Identify the lewis structure resonance forms for po4 3- that minimizes formal charges. Based on this structure, what would be bond order of the phosphorous- oxygen bonds? a. ) The bond order is 2 for one, 1 for the others b. ) The bond order is 1 for all of them c. ) the bond order is 1. 5 for all of them d. ) the bond order is 5/4 for all of them e. ) none of these answer choices are correct. 5 bonds total; 4 areas ; so bond order=5/4. Which of the following would be expected to have the largest atomic/ionic radius? a. ) Rb+ c. ) kr d. ) br e. ) there is no way to predict which of these would have the largest radius. Rb+ and kr have same number of electrons, Rb have more atomic number- more force- smaller. Nh4+ -- nh3+ h+ weak acid but complete dissociate, so also a strong electrolyte.