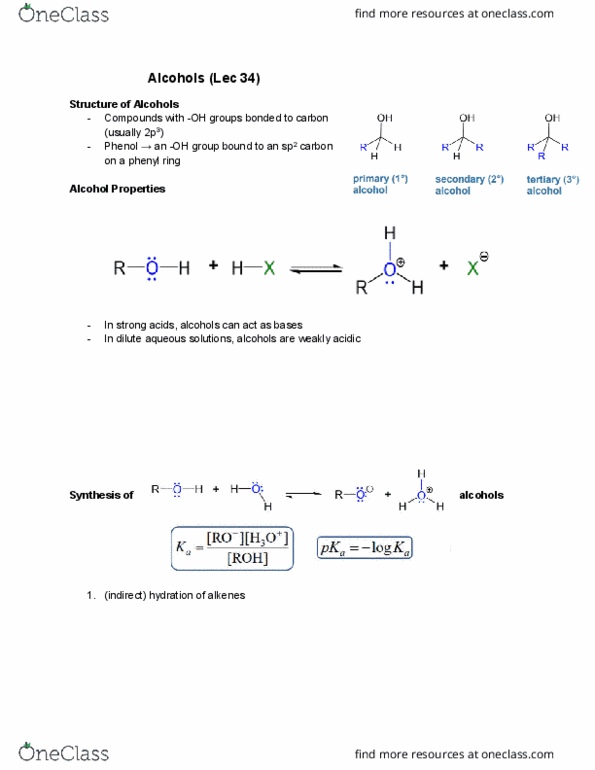
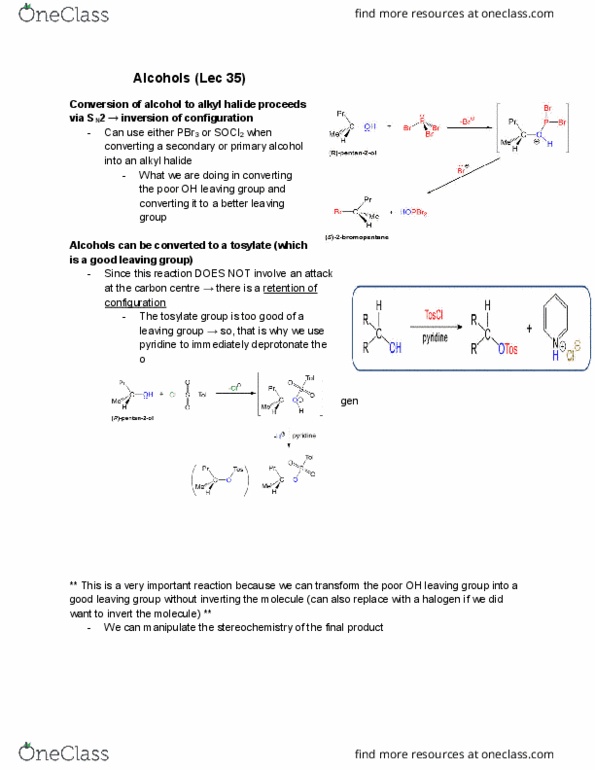
Nucleophilic Substitution â Synthesis of an Alkyl Halide
Introduction
Alkyl halides can be prepared from alcohols by reactions with hydrogen halides (HCl, HBr, or HI) via nucleophilic substitution. In this type of reaction, the nucleophile displaces a leaving group from a carbon atom of an organic substrate (here the alcohol once protonated). Both electrons of the new bond to the carbon are provided by the nucleophile while the leaving group departs with both electrons of its bond to the substrate. There are two limiting mechanisms that are most common in describing nucleophilic substitution reactions at sp3 carbon centres : (1) a direct displacement (SN2) process and (2) a process that generates an intermediate carbocation (SN1). Which mechanism is a better description depends on the structure of the molecule containing the leaving group, the nature of the leaving group, the size of the nucleophile, and the polarity of the solvent. This experiment is a near-perfect example of an SN1 substitution. H3C OH CH3 H3C + HCl H3C Cl CH3 H3C + H2O (Equation 1) In this reaction, you will prepare 2-chloro-2-methylpropane (t-butyl chloride) from 2- methyl-2-propanol (t-butyl alcohol) using HCl as the strong acid and chloride source. Note that HCl first activates the alcohol in an acid/base reaction before the SN1 reaction proceeds. The final product is characterized by its yield, boiling point, and density. The presence of a halide atom is confirmed with a silver nitrate test.
1) State which of the electrophiles given below will react preferentially by i) SN1, ii) by SN2, or iii) capable of reacting by either of the two mechanisms depending on the given conditions. How can you affect those conditions to favour SN1 or SN2? Reason your predictions based on the structures of the compounds. Br-CH3, Br-CH2CH3, Br-CH(CH3)2, Br-C(CH3)3, Br-CH2-C5H6; C5H6 = phenyl
2) Rank the nucleophiles given below based on high, mediocre, and low nucleophilicity and reason why based on their structures. HO-CH3, H2N-CH3, KO-CH3, KO-C(CH3)3
3) Name two solvents that are commonly used for SN2 reactions. because they considerably slow down SN1 reactions.
4) Can you ever have only SN2 or only SN1?
5) Iodine is a better leaving group than bromine. But iodine is a better nucleophile than bromine. Why is that?
6) Using orbitals, draw the transition state of the SN2 reaction between sodium cyanide and bromomethane. Which orbitals are interacting with which? Why is the bond breaking? Why is the orientation important? Is there another angle besides backside attack that could theoretically work based on orbital geometry? Why doesnât this happen?
7) Name four reagents you can use to dry solvent. Why do we use magnesium sulfate in this lab instead of them?
8) Draw a mechanism with all the proper arrows for the reaction from this experiment.
9) What are we removing with the water wash? Why donât we just add the sodium bicarbonate directly to the reaction mixture instead of doing a water wash?