BCH210H1 Lecture Notes - Lecture 5: Alpha And Beta Carbon, Optical Rotation, Carboxylic Acid
BCH210H1 verified notes
5/39View all
Document Summary
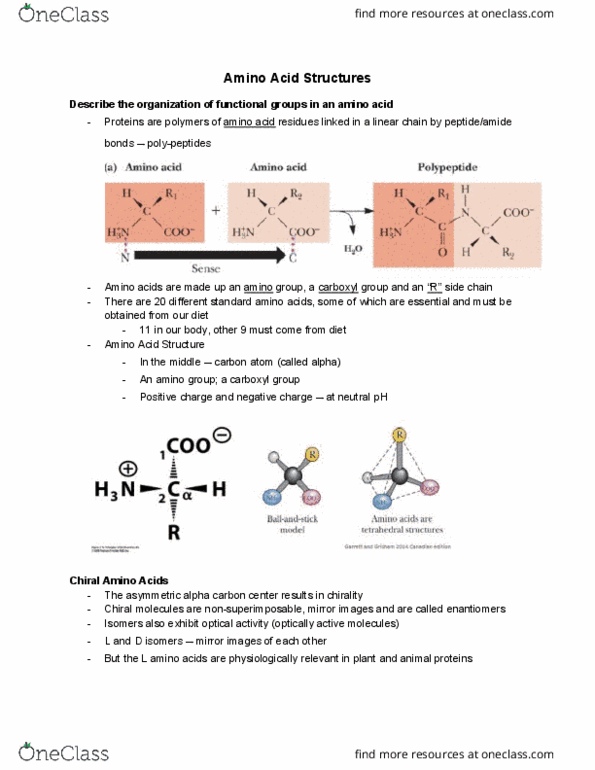
Describe the organization of functional groups in an amino acid. Proteins are polymers of amino acid residues linked in a linear chain by peptide/amide bonds poly-peptides. Amino acids are made up an amino group, a carboxyl group and an r side chain. There are 20 different standard amino acids, some of which are essential and must be obtained from our diet. 11 in our body, other 9 must come from diet. In the middle carbon atom (called alpha) Positive charge and negative charge at neutral ph. Isomers also exhibit optical activity (optically active molecules) The asymmetric alpha carbon center results in chirality. Chiral molecules are non-superimposable, mirror images and are called enantiomers. L and d isomers mirror images of each other. But the l amino acids are physiologically relevant in plant and animal proteins. Definition a zwitterion is a neutral molecule that has separate positively and negatively charged functional groups. Amino acids are overall neutral ph 7; net charge 0.