CHEM 1210 Chapter Notes - Chapter 4: Molar Concentration, Triethylene Glycol, Chemical Equation

37
CHEM 1210 Full Course Notes
Verified Note
37 documents
Document Summary
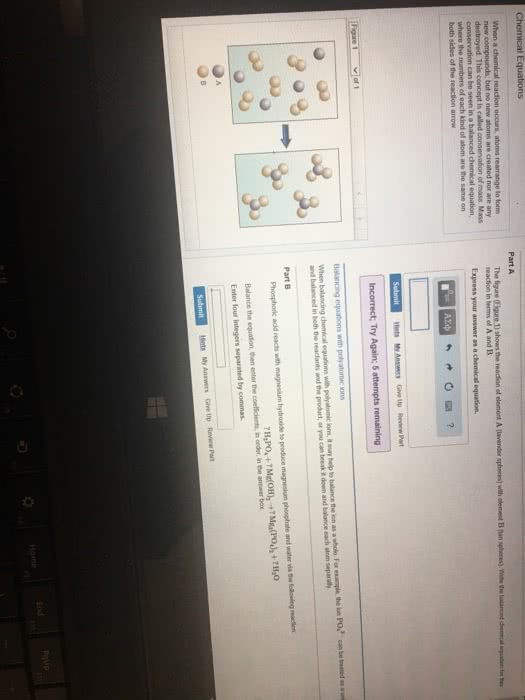
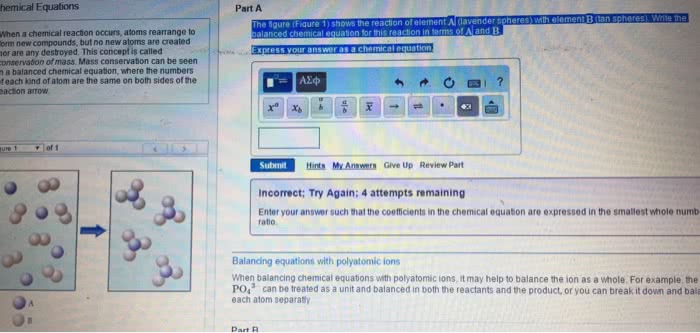
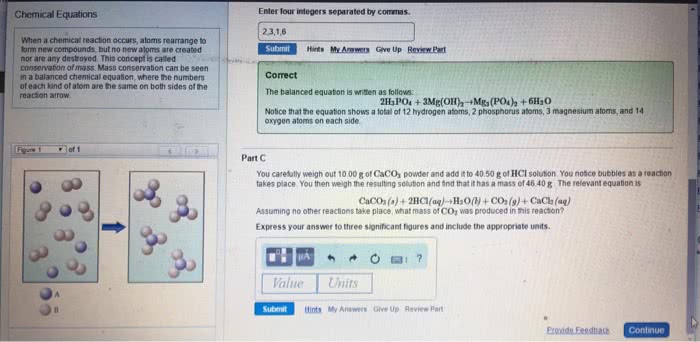
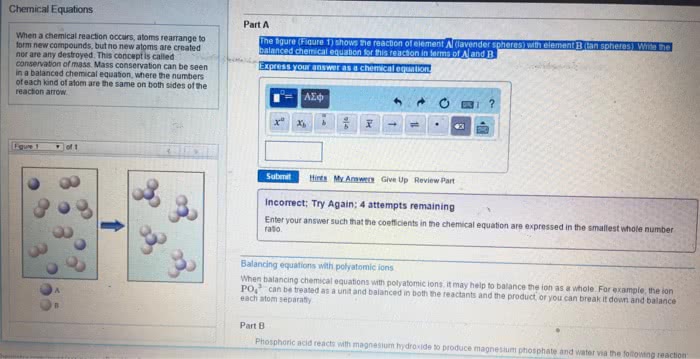
Chemical reaction process where reactants are converted into products. Balanced equation - number of atoms of each element is equal on both sides of the equation. Can include states of matter in chemical equation gas (g), liquid (l), solid (s), aqueous (aq) Reaction occurs at 350 c, under gas pressure 340 x normal atm pressure, with zno and cr2o3 as catalysts. Stoichiometry relationships or ratios between substances undergoing a chemical or physical change. Stoichiometric factor mole amounts of any two substances involved in a chemical reaction (mole ratio) Example: what mass of co2 is formed in the reaction of 4. 16g triethylene glycol, Co2 = 4. 16g c6h14o4 x x x = 7. 31g co2. Mivi = mfvf i initial undiluted solution f final diluted solution n amount of solute (in moles) Stoichiometric proportions when all of the reactants are completely and simultaneously consumed in a chemical reaction.