Chemistry 1024A/B Chapter 6: Chem1024b_2013_chapter06.pdf
Document Summary
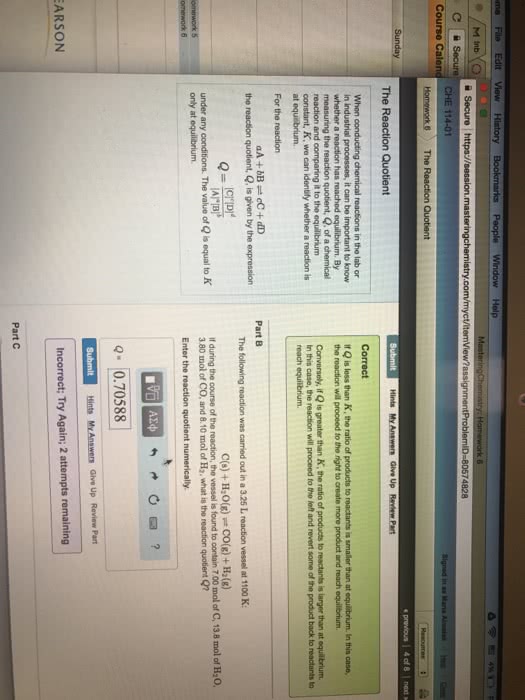
Consider a chemical reaction, e. g. a b (1) So far we have mostly assumed that chemical reactions can only occur in one direction. However, realistically also the reverse reaction can occur. Will [a] go down to zero: no. Will [b] go down to zero: no. According to (1), there will always be some. We express the fact that the reaction can proceed in both directions by using the . This is read as a is in equilibrium with b . Because the reaction can proceed in both directions, an equilibrium will be established, where the concentrations [a] and [b] are in a fixed ratio. We call this ratio the equilibrium constant k. Example: assume k = 1, i. e. at equilibrium there will always be [b] = [a]. Consider the following cases. initial concentration (m) equilibrium (= final) concentration (m) The value of k depends on the chemical nature of reactants and products, and on the temperature.