10
answers
2
watching
605
views
3 Nov 2021
The following figure represents solutions that occur at various stages during the titration of a weak acid, HAHA, with NaOHNaOH. (The Na+Na+ ions and water have been omitted for clarity.) Which of the following statements are true?
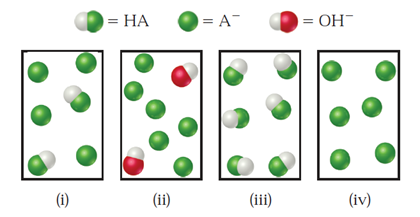
(i) The equivalence point is represented in (iv).
(ii) Both (i) and (ii) are buffer solutions.
(iii) The highest pHpH is represented by (ii).
The following figure represents solutions that occur at various stages during the titration of a weak acid, HAHA, with NaOHNaOH. (The Na+Na+ ions and water have been omitted for clarity.) Which of the following statements are true?

(i) The equivalence point is represented in (iv).
(ii) Both (i) and (ii) are buffer solutions.
(iii) The highest pHpH is represented by (ii).
christopherc63Lv10
26 Nov 2022
Read by 1 person
Read by 1 person
Read by 1 person
24 Dec 2021
Already have an account? Log in
Read by 1 person
19 Dec 2021
Already have an account? Log in
Read by 1 person
pentest1820Lv10
16 Dec 2021
Already have an account? Log in
Read by 1 person
Read by 1 person
Read by 1 person
suandrip3827Lv10
4 Nov 2021
Already have an account? Log in
Read by 1 person
christ5022Lv4
4 Nov 2021
Already have an account? Log in