1.78 Bond Angle in Methane. In the methane molecule, CH4, each hydrogen atom is at a corner of a regular tetrahedron with the carbon atom at the center. In coordinates for which one of the C-H bonds is in the direction of î + + k, an adjacent C-H bond is in the î- ì - k direction. Calculate the angle be- tween these two bonds.
For unlimited access to Homework Help, a Homework+ subscription is required.
Related textbook solutions
Related questions
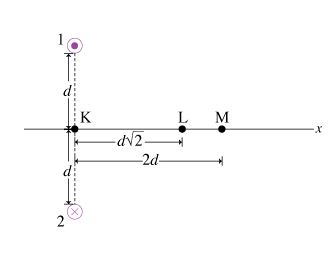
In this problem, you will be asked to calculate the magnetic field due to a set of two wires with antiparallel currents as shown in the diagram (Figure 1) . Each of the wires carries a current of magnitude I. The current in wire 1 is directed out of the page and that in wire 2 is directed into the page. The distance between the wires is 2d. The x axis is perpendicular to the line connecting the wires and is equidistant from the wires.
As you answer the questions posed here, try to look for a pattern in your answers.
Part A
Which of the vectors best represents the direction of the magnetic field created at point K (see the diagram in the problem introduction) by wire 1 alone?
Enter the number of the vector with the appropriate direction.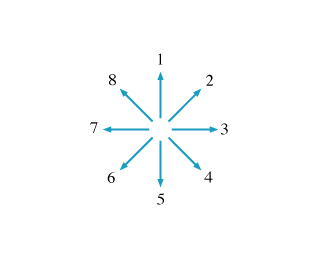
Part B
Which of the vectors best represents the direction of the magnetic field created at point K by wire 2 alone?
Enter the number of the vector with the appropriate direction.
Part C
Which of these vectors best represents the direction of the net magnetic field created at point K by both wires?
Enter the number of the vector with the appropriate direction.
Part D
Point M is located a distance 2d from the midpoint between the two wires. Find the magnitude of the magnetic field B1M created at point M by wire 1.
Express your answer in terms of I, d, and appropriate constants.
|
|
|||
| B1M= |
Part E
Find the magnitude of the net magnetic field BM created at point M by both wires.
Express your answer in terms of I, d, and appropriate constants.
|
|
|||
| BM= |



