2
answers
0
watching
4
views
13 Dec 2019
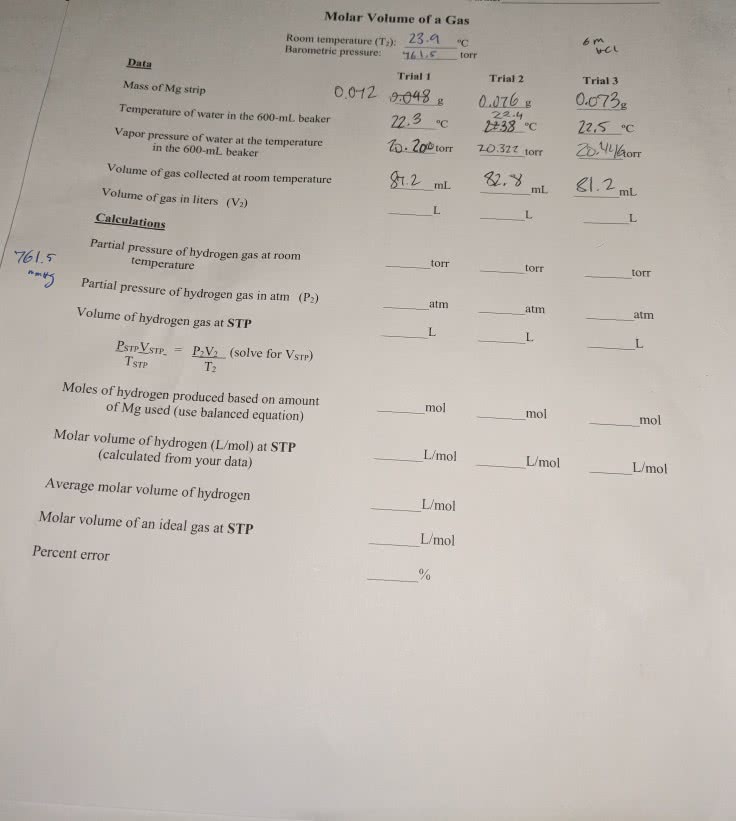
0.727 g of an unknown metal is reacted with excess concentrated hydrochloric acid according to the reaction below. 0.335 L of hydrogen gas was collected over water on a day when the temperature was 25.0 oC and the pressure was 0.98 atm. The vapor pressure of water at 25.0 oC is 23.8 torr. Report the partial pressure of hydrogen gas in atmospheres, the molar mass of the metal and the atomic symbol for the metal. The ideal gas law constant R = 0.0821 L*atm*K-1mol-1.
0.727 g of an unknown metal is reacted with excess concentrated hydrochloric acid according to the reaction below. 0.335 L of hydrogen gas was collected over water on a day when the temperature was 25.0 oC and the pressure was 0.98 atm. The vapor pressure of water at 25.0 oC is 23.8 torr. Report the partial pressure of hydrogen gas in atmospheres, the molar mass of the metal and the atomic symbol for the metal. The ideal gas law constant R = 0.0821 L*atm*K-1mol-1.
ayush0047Lv5
31 Mar 2024
Unlock all answers
Get 1 free homework help answer.
Already have an account? Log in
Collen VonLv2
16 Dec 2019
Get unlimited access
Already have an account? Log in