2
answers
1
watching
367
views
7 Apr 2021
A chemist fills a reaction vessel with 0.875 atm chlorine Cl2 gas, 9.24 atm phosphorus P4 gas, and 6.89 atm phosphorus trichloride PCl3 gas at a temperature of 25.0°C.Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction:
+6Cl2gP4g 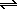 4PCl3g
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.
4PCl3g
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.
A chemist fills a reaction vessel with 0.875 atm chlorine Cl2 gas, 9.24 atm phosphorus P4 gas, and 6.89 atm phosphorus trichloride PCl3 gas at a temperature of 25.0°C.Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction:
+6Cl2gP4g 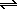 4PCl3g
4PCl3g
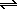 4PCl3g
4PCl3g
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.
Read by 1 person
2 Jun 2021
Already have an account? Log in


