1
answer
0
watching
483
views
cyanwolf470Lv1
14 Mar 2020
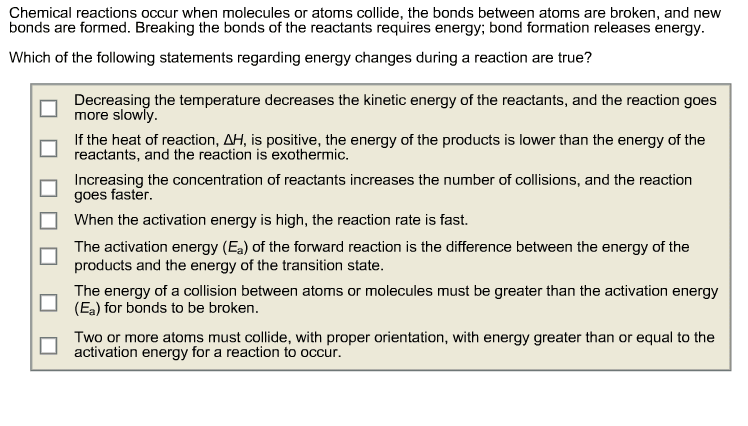
Indicate whether each statement is true or false.
(a) If you compare two reactions with similar collision factors, the one with the larger activation energy will be faster.
(b) A reaction that has a small rate constant must have a small frequency factor.
(c) Increasing the reaction temperature increases the fraction of successful collisions between reactants.
Indicate whether each statement is true or false.
(a) If you compare two reactions with similar collision factors, the one with the larger activation energy will be faster.
(b) A reaction that has a small rate constant must have a small frequency factor.
(c) Increasing the reaction temperature increases the fraction of successful collisions between reactants.
1
answer
0
watching
483
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Reid WolffLv2
28 May 2020
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232