1
answer
0
watching
195
views
graygnat657Lv1
22 Mar 2020
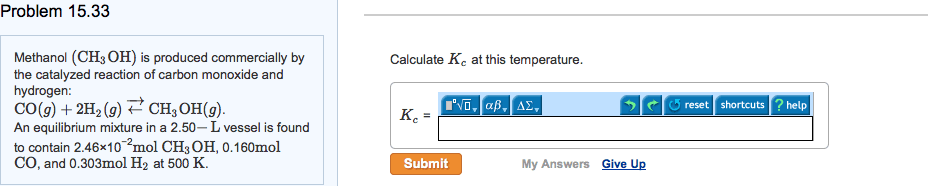
Methanol (CH3OH) is produced commercially by the catalyzed reaction of carbon monoxide and hydrogen: CO(g) + 2 H2(g)  CH3OH(g). An equilibrium mixture in a 2.00-L vessel is found to contain 0.0406 mol CH3OH, 0.170 mol CO, and 0.302 mol H2 at 500 K. Calculate Kc at this temperature.
CH3OH(g). An equilibrium mixture in a 2.00-L vessel is found to contain 0.0406 mol CH3OH, 0.170 mol CO, and 0.302 mol H2 at 500 K. Calculate Kc at this temperature.
Methanol (CH3OH) is produced commercially by the catalyzed reaction of carbon monoxide and hydrogen: CO(g) + 2 H2(g) CH3OH(g). An equilibrium mixture in a 2.00-L vessel is found to contain 0.0406 mol CH3OH, 0.170 mol CO, and 0.302 mol H2 at 500 K. Calculate Kc at this temperature.
Bunny GreenfelderLv2
29 May 2020