1
answer
0
watching
251
views
purplepug53Lv1
1 Mar 2020
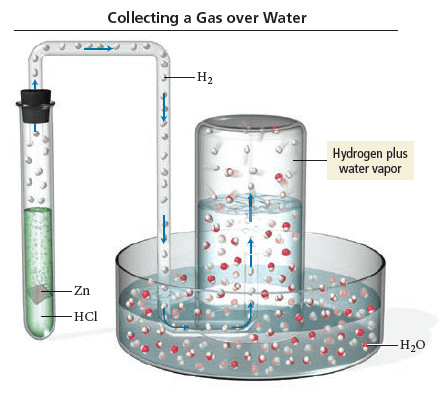
A common way to make hydrogen gas in the laboratory is to place a metal such as zinc in hydrochloric acid (see the figure). The hydrochloric acid reacts with the metal to produce hydrogen gas, which is then collected over water. Suppose a student carries out this reaction and collects a total of 140.2 mL of gas at a pressure of 749 mmHg and a temperature of 25∘C. What mass of hydrogen gas (in mg) does the student collect? (The vapor pressure of water is 23.78 mmHg at 25∘C.)
A common way to make hydrogen gas in the laboratory is to place a metal such as zinc in hydrochloric acid (see the figure). The hydrochloric acid reacts with the metal to produce hydrogen gas, which is then collected over water. Suppose a student carries out this reaction and collects a total of 140.2 mL of gas at a pressure of 749 mmHg and a temperature of 25∘C. What mass of hydrogen gas (in mg) does the student collect? (The vapor pressure of water is 23.78 mmHg at 25∘C.)