0
answers
0
watching
161
views
18 Dec 2019
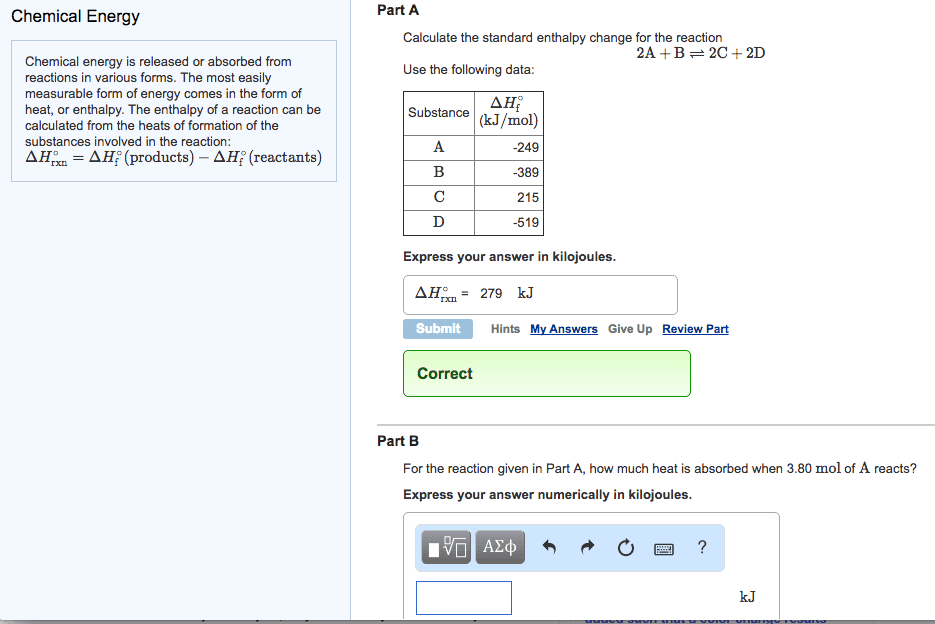
Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction:
ÎHârxn=Σ npÎHâf(products)âΣ nrÎHâf(reactants)
where n represents the stoichiometric coefficients.
Calculate the standard enthalpy change for the reaction
2A+Bâ2C+2D
Use the following data:
Substance ÎHâf
(kJ/mol) A -231 B -407 C 219 D -497
Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction:
ÎHârxn=Σ npÎHâf(products)âΣ nrÎHâf(reactants)
where n represents the stoichiometric coefficients.
Calculate the standard enthalpy change for the reaction
2A+Bâ2C+2D
Use the following data:
| Substance | ÎHâf (kJ/mol) |
| A | -231 |
| B | -407 |
| C | 219 |
| D | -497 |