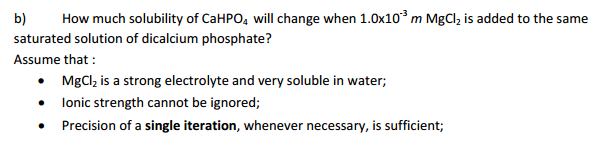1
answer
0
watching
174
views
13 Dec 2019
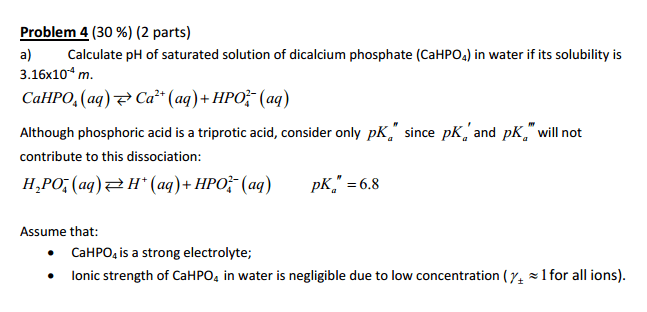
Dibasic calcium phosphate (CaHPO4) has limited solubility in water with solubility product of Ksp= 7.23*10^-7. Taking into account ionic interactions, what will be the concentration of Ca2+ ions in the solution saturated with CaHPO4 after 2.5*10^-3 m magnesium chloride (MgCl2) is dissolved in the same solution.
Dibasic calcium phosphate (CaHPO4) has limited solubility in water with solubility product of Ksp= 7.23*10^-7. Taking into account ionic interactions, what will be the concentration of Ca2+ ions in the solution saturated with CaHPO4 after 2.5*10^-3 m magnesium chloride (MgCl2) is dissolved in the same solution.
1
answer
0
watching
174
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Lelia LubowitzLv2
17 Dec 2019
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232