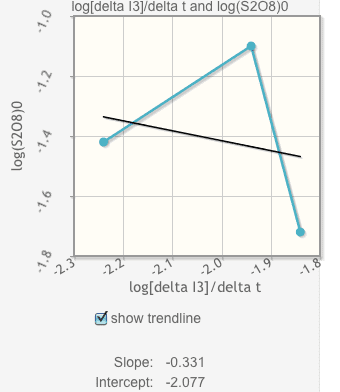
A student studies the kinetics of reaction 1 below using a clock reaction technique, reaction 2, and the procedures given in this experiment. For reaction mixture 1, he measures a time to color change of 121 seconds and for reaction mixture 2, he measures a time of 58 seconds. The temperatures of the reaction mixtures were the same for both experiments.
6Iâ (aq)+ BrO3â (aq) + 6H+(aq) --> 3I2 (aq) + Brâ (aq) + 3H2O(l) (Reaction1,slow)
I2 (aq) + 2 S2O32â (aq) --> 2 Iâ (aq) + S4O62â (aq) (Reaction 2, fast)
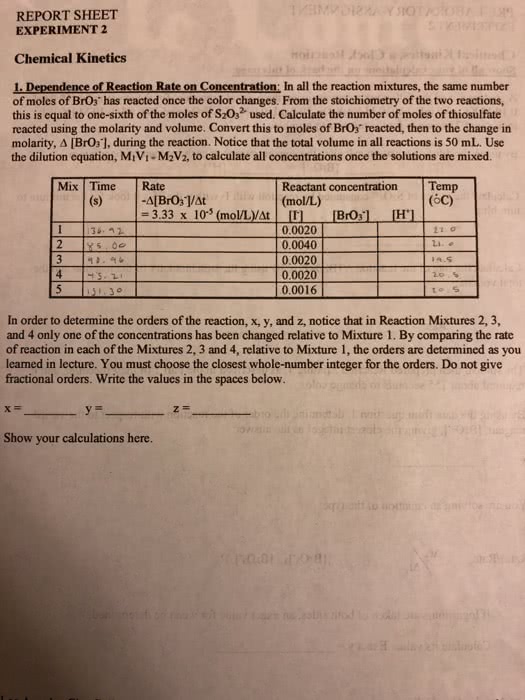
a. Determine the change in concentration of S2O32â that occurred during the time period measured. This should be the same for both the reaction mixtures. First, use M1V1=M2V2 to determine the initial concentration of the S2O32â after mixing the contents of the two flasks but before the reaction begins. Next, find the final concentration of S2O32â by remembering that all the S2O32â is consumed when the color change occurs. You can then find the change in the concentration of S2O32â.
[S2O32â]0 =
â[S2O32â] =
b. Use the stoichiometry of Reactions 1 and 2 to determine the change in concentration of BrO3â during
the same time period. â[BrO3â] =
c. Calculate the initial rate for each reaction mixture. Recall that Rate = â â[BrOâ ] / ât
Rate for mixture 1:
Rate for mixture 2:
d. Method of Initial Rates
Complete the table below using M1V1=M2V2 to find the initial concentration of each reactant after mixing the contents of flasks I and II for both reaction mixtures. Enter the initial rates computed above.
Rxn Mixture [Iâ]0(M) [BrO3â]0(M) [H+]0 (M) Rate (M/s)
1
2
Use the method of initial rates and the relevant data above to determine the order of reaction 1 with respect to the concentration of iodide ions.
Order with respect to [Iâ]:_________________________
e. It is critical for the success of this experiment that the concentrations of the reactants Iâ, BrO3â, and H+ do not change significantly over the time period measured.
Why is this important?
f. By what percent did the bromate ion concentration change during the time period considered for mixture 1 in this experiment?
PercentChange = (|â[BrO3â ]| / [BrO3â ]0 ) x 100%
A student studies the kinetics of reaction 1 below using a clock reaction technique, reaction 2, and the procedures given in this experiment. For reaction mixture 1, he measures a time to color change of 121 seconds and for reaction mixture 2, he measures a time of 58 seconds. The temperatures of the reaction mixtures were the same for both experiments.
6Iâ (aq)+ BrO3â (aq) + 6H+(aq) --> 3I2 (aq) + Brâ (aq) + 3H2O(l) (Reaction1,slow)
I2 (aq) + 2 S2O32â (aq) --> 2 Iâ (aq) + S4O62â (aq) (Reaction 2, fast)
a. Determine the change in concentration of S2O32â that occurred during the time period measured. This should be the same for both the reaction mixtures. First, use M1V1=M2V2 to determine the initial concentration of the S2O32â after mixing the contents of the two flasks but before the reaction begins. Next, find the final concentration of S2O32â by remembering that all the S2O32â is consumed when the color change occurs. You can then find the change in the concentration of S2O32â.
[S2O32â]0 =
â[S2O32â] =
b. Use the stoichiometry of Reactions 1 and 2 to determine the change in concentration of BrO3â during
the same time period. â[BrO3â] =
c. Calculate the initial rate for each reaction mixture. Recall that Rate = â â[BrOâ ] / ât
Rate for mixture 1:
Rate for mixture 2:
d. Method of Initial Rates
Complete the table below using M1V1=M2V2 to find the initial concentration of each reactant after mixing the contents of flasks I and II for both reaction mixtures. Enter the initial rates computed above.
Rxn Mixture [Iâ]0(M) [BrO3â]0(M) [H+]0 (M) Rate (M/s)
1
2
Use the method of initial rates and the relevant data above to determine the order of reaction 1 with respect to the concentration of iodide ions.
Order with respect to [Iâ]:_________________________
e. It is critical for the success of this experiment that the concentrations of the reactants Iâ, BrO3â, and H+ do not change significantly over the time period measured.
Why is this important?
f. By what percent did the bromate ion concentration change during the time period considered for mixture 1 in this experiment?
PercentChange = (|â[BrO3â ]| / [BrO3â ]0 ) x 100%