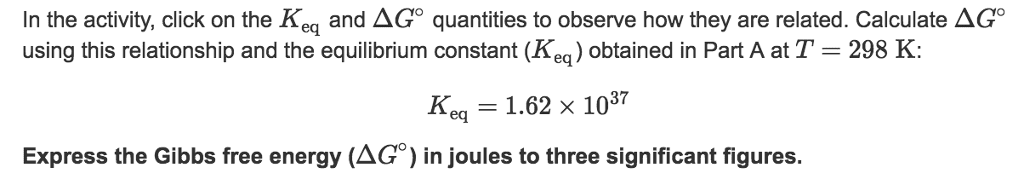1
answer
0
watching
63
views
13 Dec 2019
In the activity, click on the Keq and ÎGâ quantities to observe how they are related. Calculate ÎGâ using this relationship and the equilibrium constant (Keq) obtained in Part A at T=298K:
Keq=1.92Ã1026
Express the Gibbs free energy (ÎGâ) in joules to three significant figures.
In the activity, click on the Keq and ÎGâ quantities to observe how they are related. Calculate ÎGâ using this relationship and the equilibrium constant (Keq) obtained in Part A at T=298K:
Keq=1.92Ã1026
Express the Gibbs free energy (ÎGâ) in joules to three significant figures.
Lelia LubowitzLv2
17 Dec 2019