Please check my answers and let me know if they correct. Thanks
Exercise 1: Determining the Concentration of Acetic Acid
Data Table 1. NaOH Titration Volume.
Initial NaOH Volume (mL)
Final NaOH Volume (mL)
Total volume of NaOH used (mL)
Trial 1
9 ml
0
9
Trial 2
9 ml
0.2
8.8
Trial 3
9 ml
0.3
8.7
Average Volume of NaOH Used (mL) : 8.8
Data Table 2. Concentration of CH3COOH in Vinegar.
Average volume of NaOH used (mL)
Concentration CH3COOH in vinegar (mol/L)
% CH3COOH in vinegar
8.8
72 mol/L
83%
Questions
The manufacturer of the vinegar used in the experiment stated that the vinegar contained 5.0% acetic acid. What is the percent error between your result and the manufacturerâs statement?
The acetic acid in vinegar for my experiment is 83% which is 1560% difference from the actual 5%.
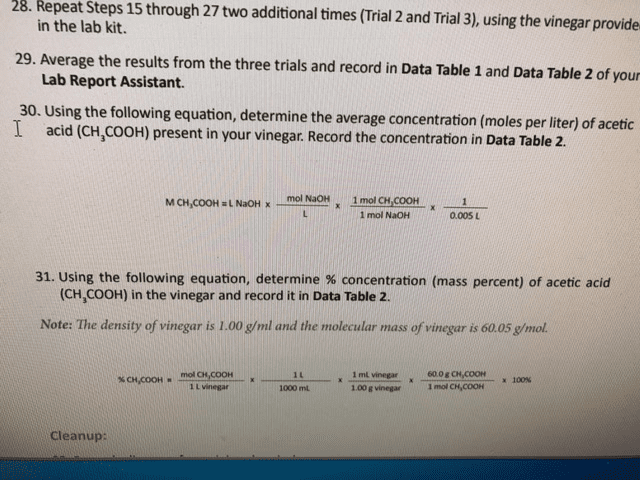
What challenges would you encounter with the titration if you had used apple cider vinegar or balsamic vinegar as the analyte instead of white vinegar?
The white vinegar is made up of pure vinegar so the results will be more accurate than the apple cider vinegar or balsamic vinegar, the later has other "impurities" in them. The apple and balsamic has other properties which will interfere with the titration.
How would your results have differed if the tip of the titrator was not filled with NaOH before the initial volume reading was recorded? Explain your answer.
If the tip of the titration had not been filled with NaOH, the results would have been inaccurate because the tip contains about 0.5ml-1ml of volume. Priming the tip is important to ensure accurate results.
How would your results have differed if you had over-titrated (added drops of NaOH to the analyte beyond the stoichiometric equivalence point)?
If I had over-titrated, the percent composition of acetic acid would be greater in my results since my results would have indicated that it took a larger volume of NaOH to neutralize the acetic acid in vinegar.
If a 7.0 mL sample of vinegar was titrated to the stoichiometric equivalence point with 7.5 mL of 1.5M NaOH, what is the mass percent of CH3COOH in the vinegar sample?
Not enough information! need the density of vinegar.
Why is it important to do multiple trials of a titration, instead of only one trial?
If only one trial is run, the results may not be entirely accurate and may contain mechanical or calculation errors. Running multiple trials helps ensure that no errors are made in the results and you have several results to compare to.
Please check my answers and let me know if they correct. Thanks
Exercise 1: Determining the Concentration of Acetic Acid
Data Table 1. NaOH Titration Volume.
| Initial NaOH Volume (mL) | Final NaOH Volume (mL) | Total volume of NaOH used (mL) | |
| Trial 1 | 9 ml | 0 | 9 |
| Trial 2 | 9 ml | 0.2 | 8.8 |
| Trial 3 | 9 ml | 0.3 | 8.7 |
| Average Volume of NaOH Used (mL) : 8.8 | |||
Data Table 2. Concentration of CH3COOH in Vinegar.
| Average volume of NaOH used (mL) | Concentration CH3COOH in vinegar (mol/L) | % CH3COOH in vinegar |
| 8.8 | 72 mol/L | 83% |
Questions
The manufacturer of the vinegar used in the experiment stated that the vinegar contained 5.0% acetic acid. What is the percent error between your result and the manufacturerâs statement?
The acetic acid in vinegar for my experiment is 83% which is 1560% difference from the actual 5%.
What challenges would you encounter with the titration if you had used apple cider vinegar or balsamic vinegar as the analyte instead of white vinegar?
The white vinegar is made up of pure vinegar so the results will be more accurate than the apple cider vinegar or balsamic vinegar, the later has other "impurities" in them. The apple and balsamic has other properties which will interfere with the titration.
How would your results have differed if the tip of the titrator was not filled with NaOH before the initial volume reading was recorded? Explain your answer.
If the tip of the titration had not been filled with NaOH, the results would have been inaccurate because the tip contains about 0.5ml-1ml of volume. Priming the tip is important to ensure accurate results.
How would your results have differed if you had over-titrated (added drops of NaOH to the analyte beyond the stoichiometric equivalence point)?
If I had over-titrated, the percent composition of acetic acid would be greater in my results since my results would have indicated that it took a larger volume of NaOH to neutralize the acetic acid in vinegar.
If a 7.0 mL sample of vinegar was titrated to the stoichiometric equivalence point with 7.5 mL of 1.5M NaOH, what is the mass percent of CH3COOH in the vinegar sample?
Not enough information! need the density of vinegar.
Why is it important to do multiple trials of a titration, instead of only one trial?
If only one trial is run, the results may not be entirely accurate and may contain mechanical or calculation errors. Running multiple trials helps ensure that no errors are made in the results and you have several results to compare to.