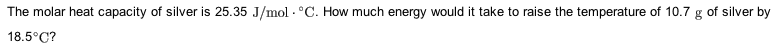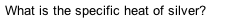Heat Capacity
Learning Goal:
To understand the concepts of heat capacity, specific heat, and molar heat capacity.
Heat capacity, C, is the amount of energy required to raise the temperature of a substance by exactly 1 degree Celsius. The energy needed to warm an object increases as the mass of that object increases. We see this in our everyday life. For example, we know that it takes much more energy to heat a large tank of water than a small cup. Because of this dependence on mass, experimentally determined heat capacities are always reported in terms of the amount of the substance that is heated. One method is to report how much energy it takes to raise the temperature of one mole of a substance by exactly 1 degree Celsius. This value is the molar heat capacity, which has the symbol Cp.The molar heat capacity is given in the units J/(molâ âC). A second method is to report how much energy it takes to raise the temperature of one gram of a substance by exactly 1 degree Celsius. This value is the specific heat, which has been given the symbol Cs. The units for specific heat are J/(gâ âC). The heat capacity of a substance is therefore related to the energy q needed to raise its temperature by an amount ÎT. That is, q=nCpÎT, where n denotes the number of moles of the substance, or q=mCsÎT, where m denotes the number of grams of the substance.
Part A
It takes 51.0 J to raise the temperature of an 11.1 g piece of unknown metal from 13.0âC to 24.3 âC. What is the specific heat for the metal?
Express your answer with the appropriate units.
Cs =
SubmitHintsMy AnswersGive UpReview Part
Parts B and C
The next two questions pertain to silver. They have nothing to do with unknown metal described in Part A.
Part B
The molar heat capacity of silver is 25.35 J/molâ âC. How much energy would it take to raise the temperature of 11.1 g of silver by 15.8 âC?
Express your answer with the appropriate units.
q =
SubmitHintsMy AnswersGive UpReview Part
Part C
What is the specific heat of silver?
Express your answer with the appropriate units.
SubmitHintsMy AnswersGive UpReview Part
| Heat Capacity Learning Goal: To understand the concepts of heat capacity, specific heat, and molar heat capacity. Heat capacity, C, is the amount of energy required to raise the temperature of a substance by exactly 1 degree Celsius. The energy needed to warm an object increases as the mass of that object increases. We see this in our everyday life. For example, we know that it takes much more energy to heat a large tank of water than a small cup. Because of this dependence on mass, experimentally determined heat capacities are always reported in terms of the amount of the substance that is heated. One method is to report how much energy it takes to raise the temperature of one mole of a substance by exactly 1 degree Celsius. This value is the molar heat capacity, which has the symbol Cp.The molar heat capacity is given in the units J/(molâ âC). A second method is to report how much energy it takes to raise the temperature of one gram of a substance by exactly 1 degree Celsius. This value is the specific heat, which has been given the symbol Cs. The units for specific heat are J/(gâ âC).The heat capacity of a substance is therefore related to the energy q needed to raise its temperature by an amount ÎT. That is, q=nCpÎT, where n denotes the number of moles of the substance, or q=mCsÎT, where m denotes the number of grams of the substance. | Part A It takes 51.0 J to raise the temperature of an 11.1 g piece of unknown metal from 13.0âC to 24.3 âC. What is the specific heat for the metal? Express your answer with the appropriate units.
SubmitHintsMy AnswersGive UpReview Part Parts B and C The next two questions pertain to silver. They have nothing to do with unknown metal described in Part A. Part B The molar heat capacity of silver is 25.35 J/molâ âC. How much energy would it take to raise the temperature of 11.1 g of silver by 15.8 âC? Express your answer with the appropriate units.
SubmitHintsMy AnswersGive UpReview Part Part C What is the specific heat of silver? Express your answer with the appropriate units.
SubmitHintsMy AnswersGive UpReview Part |