0
answers
0
watching
79
views
11 Dec 2019
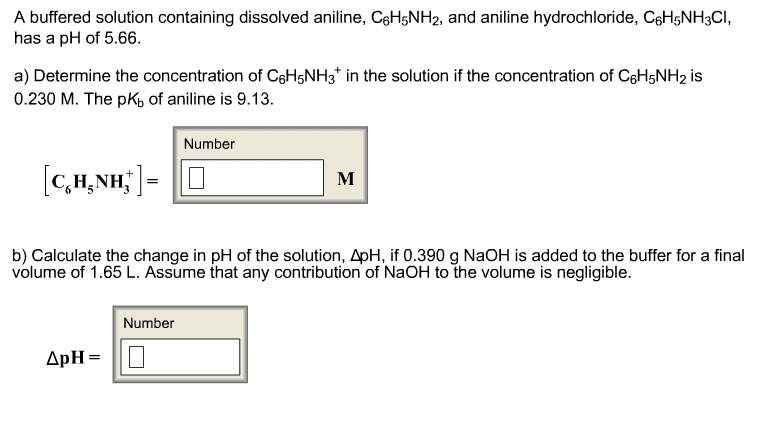
Consider a solution that contains both C6H5NH2 and C6H5NH3+. Calculate the ratio [C6H5NH2]/[C6H5NH3+] if the solution has the following pH values. (Assume that the solution is at 25°C.) (a) pH = 4.93 (b) pH = 4.70 (c) pH = 5.26 (d) pH = 5.54
Consider a solution that contains both C6H5NH2 and C6H5NH3+. Calculate the ratio [C6H5NH2]/[C6H5NH3+] if the solution has the following pH values. (Assume that the solution is at 25°C.) (a) pH = 4.93 (b) pH = 4.70 (c) pH = 5.26 (d) pH = 5.54
0
answers
0
watching
79
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232