1
answer
0
watching
94
views
27 Nov 2019
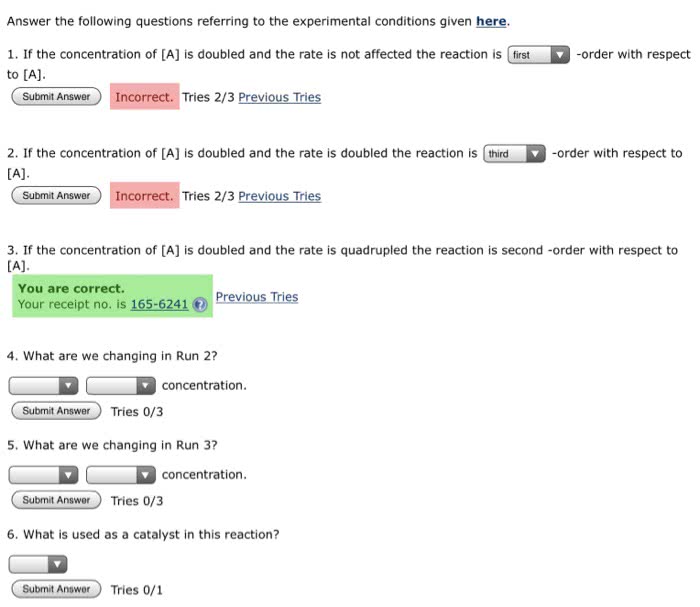
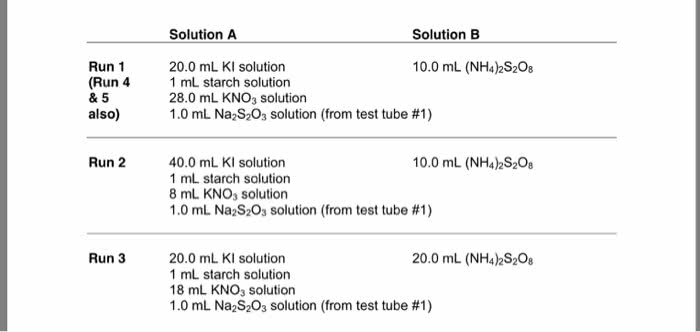
In a reaction that is first-order with respect to reactant A andsecond-order with respect to reactant B, what will happen to therate of the reaction if we
a. double the concentration of A
b. double the concentrations of A and B
c half the concentration of A and simultaneously triple of theconcentration of B
In a reaction that is first-order with respect to reactant A andsecond-order with respect to reactant B, what will happen to therate of the reaction if we
a. double the concentration of A
b. double the concentrations of A and B
c half the concentration of A and simultaneously triple of theconcentration of B
Reid WolffLv2
27 Nov 2019