The pH of a solution is measured to be 3.50. Calculate the hydrogen ion
concentration of the solution.
2) Give the conjugate acid or base of the following:
(a) conjugate base of HC2H3O2 (b) conjugate acid of CN-
(c) conjugate base of HSO4
(d) conjugate acid of CO3
-
2-
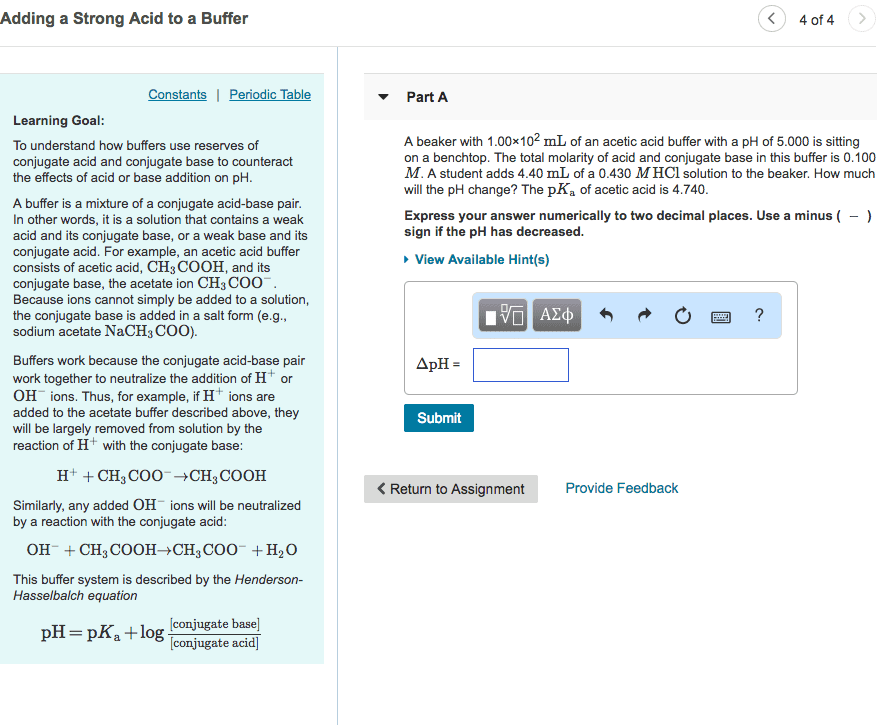
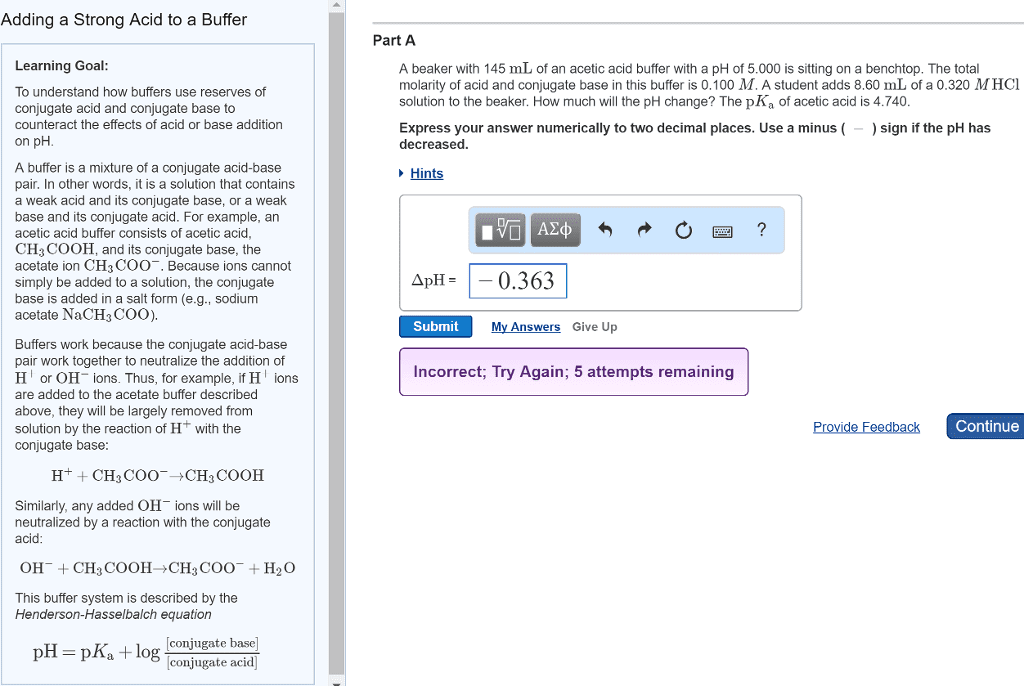
3) What is a buffer? How is a buffer prepared?
4) A buffer solution was prepared using the conjugate acid-base pair acetic acid and acetate
ions. Write the chemical equations showing the reactions that take place when
(a) H+ ions are added to the buffer solution
(b) OH- ions are added to the buffer solution
5) Write the neutralization reaction (in net ionic form) for the titration of acetic acid (CH3COOH)
with sodium hydroxide (NaOH).
6) If you have one mole of buffer that was exactly 50% acetic acid and 50% acetate ion, how much
NaOH would it take to neutralize all of the buffer?
7) When can a salt act as a buffer? Explain.
**please type answers, ty
The pH of a solution is measured to be 3.50. Calculate the hydrogen ion
concentration of the solution.
2) Give the conjugate acid or base of the following:
(a) conjugate base of HC2H3O2 (b) conjugate acid of CN-
(c) conjugate base of HSO4
(d) conjugate acid of CO3
-
2-
3) What is a buffer? How is a buffer prepared?
4) A buffer solution was prepared using the conjugate acid-base pair acetic acid and acetate
ions. Write the chemical equations showing the reactions that take place when
(a) H+ ions are added to the buffer solution
(b) OH- ions are added to the buffer solution
5) Write the neutralization reaction (in net ionic form) for the titration of acetic acid (CH3COOH)
with sodium hydroxide (NaOH).
6) If you have one mole of buffer that was exactly 50% acetic acid and 50% acetate ion, how much
NaOH would it take to neutralize all of the buffer?
7) When can a salt act as a buffer? Explain.
**please type answers, ty